Question
Question: What is the bond order of \(C{{O}_{2}}\)?...
What is the bond order of CO2?
Solution
First you should know about the total number of bonds present in the CO2 molecule and then, using the formula of bond order as;- B.O.=no. of sigma bondsTotal no. of bonds, you can easily find the bond order of the given molecule. Now solve it.
Complete step by step answer:
First let's discuss carbon dioxide. Carbon dioxide is a gas which is formed by the reaction between the two non-metals i.e. carbon and oxygen. The chemical is supposed to take place as;-
C+O2→CO2
Now coming next to the bond order. It is defined as the number of covalent bonds present between the two bonded atoms in a molecule. It is denoted as B.O. and can be found by dividing the total number present in the carbon dioxide molecule with the number of the sigma bonds present in the molecule.
Mathematically:-
B.O.=no. of sigma bondsTotal no. of bonds
Now considering the statement as;-
The structure of the carbon dioxide molecule is as;-
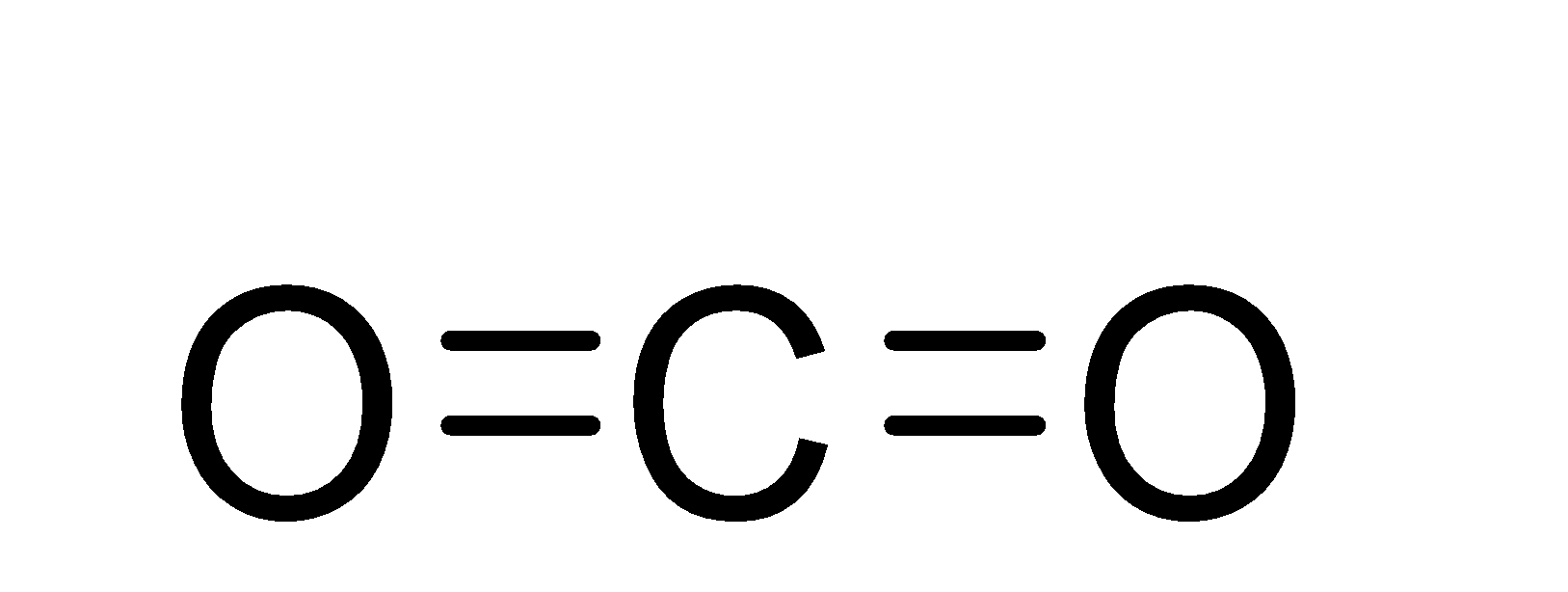
So, from the structure, we can see that there are a total number of four bonds in the carbon dioxide molecule and out of these 4 bonds, 2 are sigma bonds and two are pi-bonds.
So, the bond order of carbon dioxide molecule is as;-
B.O=24 =2
So, thus the bond order of carbon dioxide molecules is 2.
Note: The bond orders of 1,2 and 3 correspond to single, double and triple bonds but bond order can be fractional as well. The positive value of bond order indicates that the molecule is stable whereas the negative value indicates, the molecule is unstable.
