Question
Question: What is the bond line notation for trans-2-butene?...
What is the bond line notation for trans-2-butene?
Solution
Bond line notation is a convention used by organic chemists to represent molecular structures. A bond between two atoms is depicted as a line in this technique. The bound atoms' locations are shown by the line's ends. Only non-carbon and non-hydrogen atoms are specifically shown. As a result, ethane's bond line notation is . The presence of a carbon atom at either end of the line is suggested. Furthermore, because carbon has a valence of four, each carbon has three hydrogen atoms attached to it.
Complete answer:
Bond line notation is a technique of writing organic structural formulae in a quick and easy way:
The carbon and hydrogen atoms that are linked to them are not visible. Only the carbon atoms' bonds are shown as lines. The carbon atoms are represented by the vertices and ends of the lines. Any valences on carbon that aren't filled are expected to be filled by hydrogen atoms. All non-carbon atoms, as well as any hydrogen atoms linked to them, are displayed.
The acyclic alkene 2-butene has four carbon atoms. It's the simplest alkene with cis/trans-isomerism, which means it has two geometric isomers: cis-2-butene and trans-2-butene. It's a petrochemical made by catalytic cracking of crude oil or ethylene dimerization. Its primary use are in the manufacture of gasoline (petrol) and butadiene, however some 2-butene is also used to make the solvent butanone by hydration to 2-butanol and then oxidation.
Because of their close boiling points (4 ∘C for cis and 1 ∘C for trans), the two isomers are exceedingly difficult to separate by distillation. In most industrial contexts, however, separation is unnecessary since both isomers behave similarly in most of the required reactions. 70 percent (Z)-2-butene and 30% (E)-2-butene is a common industrial 2-butene combination.
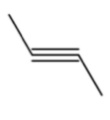
is the bond line notation for trans 2 butene.
Note:
The bond line notation approach may also be used to depict the structural formulas of organic compounds. The carbon and hydrogen atoms are not depicted in this approach; instead, only the bonds between the carbon atoms are shown as lines. The carbon atoms are represented by the ends and vertices. According to the valence laws, the amount of hydrogens must be estimated. Other atoms, besides carbon and hydrogen, are depicted.
