Question
Question: What is the bond angle of a molecule undergoing \( s{p^2} \) hybridization?...
What is the bond angle of a molecule undergoing sp2 hybridization?
Solution
Hint : VSEPR (valence shell electron pair repulsion theory) gives a basic idea about the shape of a molecule depending upon its electron pair present in the valence shell.
This concept of hybridization was first introduced by Pauling. According to Pauli, the atomic orbitals combine to form a new set of equivalent orbitals which are commonly known as hybrid orbitals.
Complete Step By Step Answer:
Hybridization is used to explain the characteristic shapes of molecules made up of 2 or more atoms.
Any molecule undergoes sp2 hybridization, one s orbital and one p orbital combine together to form sp2 hybridized orbital which are equivalent in terms of orbital energy.
For example, boron trichloride molecules undergo sp2 hybridization.
Boron acts as a central atom of the molecule with electronic configuration 1s22s22p1 .
Boron extends its valency by transferring one electron from 2s to 2p orbital.
Now the final configuration of boron becomes 1s22s12p2 with three valence.
finally, one 2s orbital and two 2p orbital of boron undergo hybridization to from sp2 hybridized
orbital.
These hybridized orbitals undergoes overlapping with 2p orbital of three chlorine atom
to form boron trichloride.
Chlorine atoms are arranged at 120∘ with the central metal atom to form a geometry known as trigonal planar.
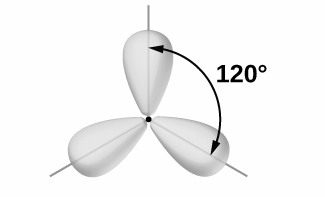
molecules undergoing sp2 hybridization includes- BF3 , AlCl3 , C2H4 , NO3− .
Hence, the bond angle of molecule undergoes sp2 Hybridization is 120 degrees.
Note :
Remember that the total number of hybridized orbitals is always a sum of all the orbital undergoes hybridization. Hybrid orbital rearrange into a specific angle to form a stable arrangement. Angle in different molecules changes with change in hybridization for example molecule undergo sp hybridization form angle of 180∘ while 109.5∘ angle is formed by sp3 hybridized molecules.
