Question
Question: What is the Bohr-Bury scheme of arrangement of electrons in an atom?...
What is the Bohr-Bury scheme of arrangement of electrons in an atom?
Solution
Electronic configuration of an atom is governed by a number of rules which includes: Aufbau principle, Bohr-Bury rule, Pauli exclusion principle and Hund’s rule. All these rules are employed to write the correct electronic configuration of any atom.
Complete answer:
Bohr-Bury rules are used to explain some important aspects of writing electronic configuration. According to this rule-
The energy of any orbital is determined with the help of numbers n and l.
The filling of electrons in the orbital takes place according to the increasing order of (n+l).
For example: energy of 3d orbital is (3+2=5), while the energy of 4s orbital is (4+0=4). Hence, according to Bohr-Bury rules energy of 3d is more than 4s orbital hence, electrons first fill in 3d orbital then fill into 4s orbital.
In case, if two orbitals possess the same energy level then the electron fills into an orbital which is associated with a lower value of n.
For example: the energy level of 2p orbital is (2+1=3), and the energy level of 3s orbital is (3+1=3). In this case the energy level of both the orbital is the same but electron first fills into 2p orbital because the value of n is smaller in 2p.
This rule helps in identifying the fact that an orbital with higher value of principal quantum number n, may have lower energy level than orbitals with lower value of principal quantum number.
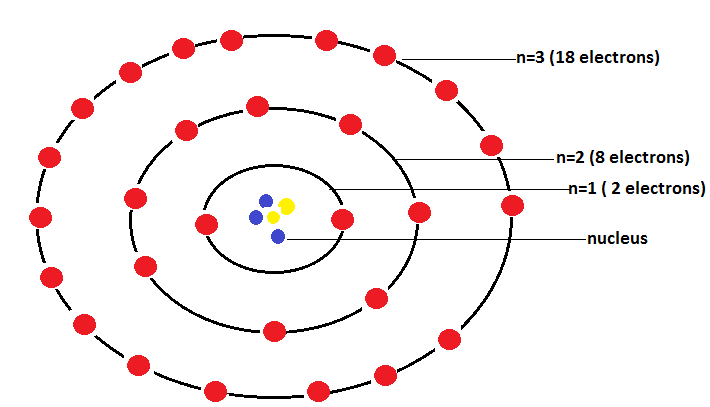
Bohr-Bury states that the maximum capacity of a shell to hold electrons in them is equal to 2n2. Where n describes the orbital energy of electrons.
Outermost shell of an atom must contain a maximum of 8 electrons to attain stability.
Electron filling proceeds to another shell until its inner shell is completely filled.
Bohr-Bury scheme of arrangement of electrons in an atom is shown as:
Note:
Aufbau helps in determining the energy of orbitals while Pauli explains the spin and maximum holding capacity of an orbital. Remember that a maximum of 2 electrons must be present in one orbital while a maximum of 8 present in orbit.
