Question
Question: What is the basicity of \[{H_3}P{O_3}\] ? A. 3 B. 2 C. 1 D. None of these...
What is the basicity of H3PO3 ?
A. 3
B. 2
C. 1
D. None of these
Solution
We need to know the dependency of the basicity of a compound. The base is a substance that can release OH− ions when dissolved in water. Similarly, an acid is a substance that can release H+ when dissolved in water. Not all bases and acids have the same ability to donate OH− and OH− ions. This ability defines the strength of a base and acid and basicity is a measure of this strength.
Complete step by step answer:
We need to know that the basicity of an acid is the number of H+ ions released, and the basicity of a base is the number of OH− ions released when dissolved in water. It determines the strength of an acid or base. The basicity of an acid depends on the number of ionizable hydrogen atoms present in the acid. The basicity of a base depends on the number of ionizable hydroxyl groups present in the base.
The given compound is H3PO3 . Clearly, it is an acid as there are no hydroxyl groups present. We now write the skeletal structure of the acid to understand the location of the hydrogen atoms to determine its basicity.
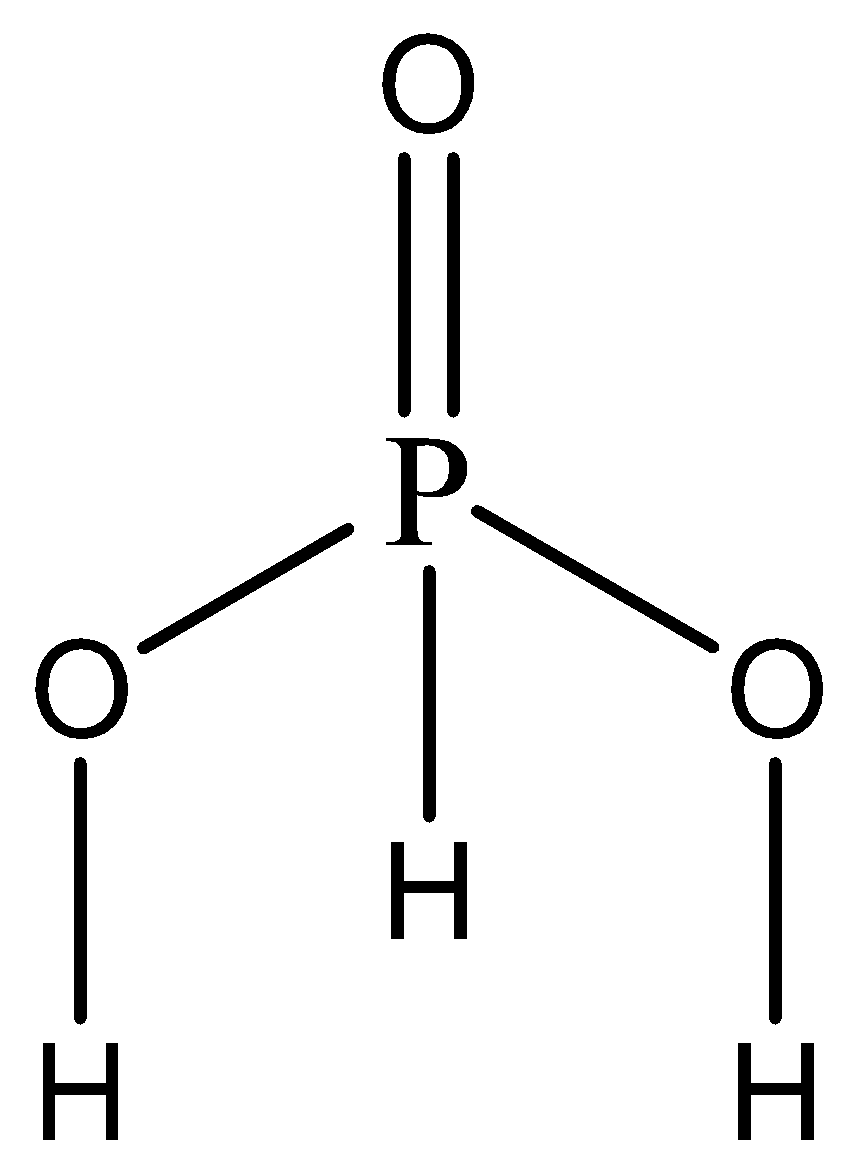
The hydrogen atoms attached directly to the oxygen atoms are ionisable. The hydrogen atom attached to the phosphorus atom is reducing in nature and non-ionisable.
Since there are two ionizable hydrogen groups in H3PO3 , hence the basicity of this acid is 2.
So, the correct answer is Option B.
Note: It must be noted that the basicity of an acid with basicity 2 is also known as dibasic acid. The acids with a single ionizable hydrogen ion are known as monobasic acid and those with three ionizable hydrogen groups are known as tribasic acids. H3PO3 is a strong reducing agent and is also known as phosphorus acid or ortho phosphorous acid.
