Question
Question: What is the B product in the following reaction? 
Solution
We will follow step to step formation of product. Li is electropositive in nature. The reaction here is organometallic reaction as metal copper is used here with organic compounds so the product formed will be organometallic.
Complete step by step answer:
In the first step a metal reacts with an organic compound. Organometallic reactions are a little different. Since metals are electropositive in nature and copper is divalent in nature it attaches two molecules of organic compound with it. This step is the formation of Gilman reagent.
In the second step it again reacts with another molecule of alkyl bromide which can be same or different and form alkane in the last i.e gilman reagent converts it into alkane.
The following is the mechanism
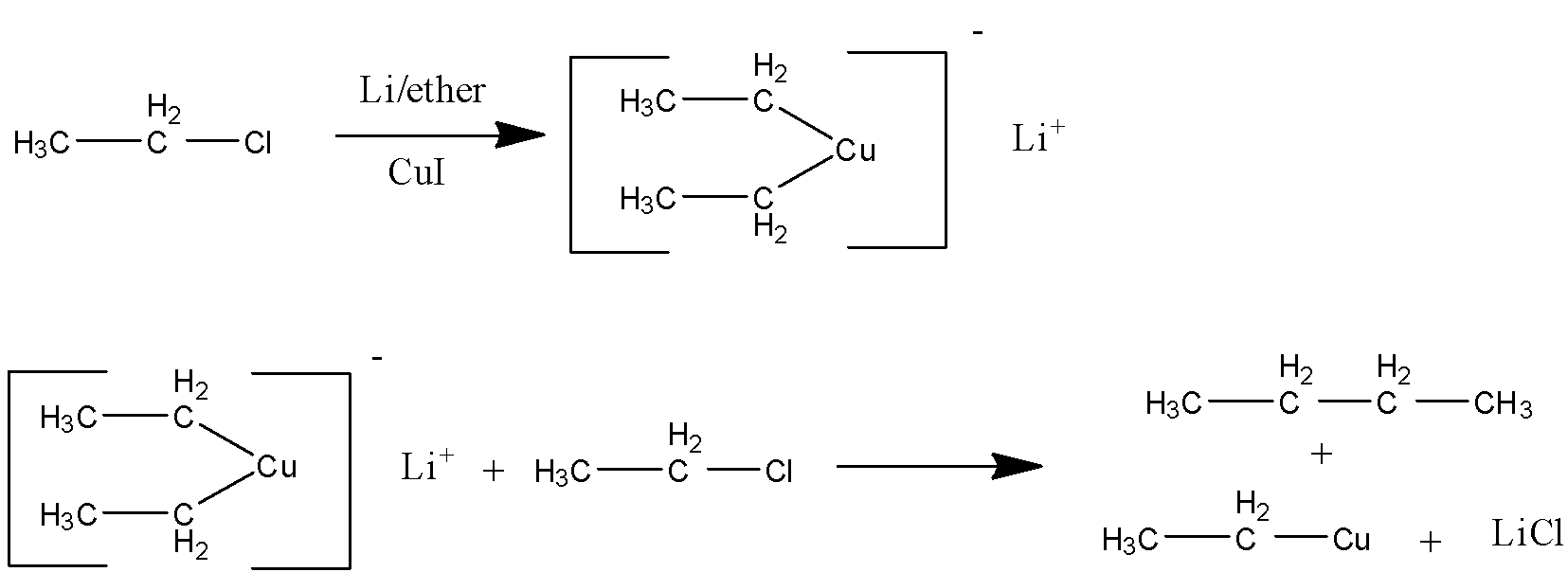
Here the product (B) is BUTANE
Gilman reagent is a di-organocopper compound which consists of lithium and copper. R2CuLi is the formula of gilman reagent here R is either aryl or alkyl group. This reagent is very important as it is different from Grignard reagent and organometallic reagent because it reacts with alkyl halides to replace the halide group with an alkyl group that can be same or different than the one inside gilman reagent.
Additional information: Organometallic reagents are reagents in which the metal is attached to organic carbon compounds. There are many organometallic reagents present, for example Grignard reagent, gilman reagent, dimethyl magnesium, organozinc compound etc.
Note:
Gilman reagent reacts with α−β−unsaturated ketone to form conjugate addition products which Grignard reagent or any other organometallic reagent cannot do. It is also a two step process just like this reaction.
