Question
Question: What is the approximate wavelength of blue light? \(\begin{aligned} & A.800A{}^\circ \\\ &...
What is the approximate wavelength of blue light?
A.800A∘B.1600A∘C.3200A∘D.4800A∘
Solution
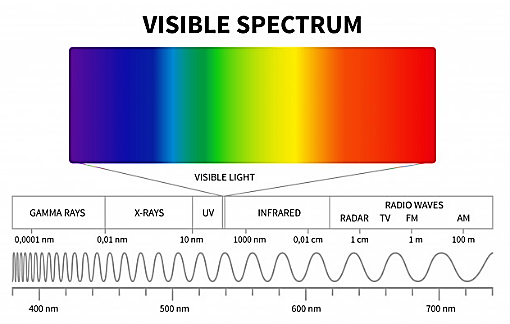
The visible white light we see is a spectrum of seven magnificent colours. The seven colours, namely Violet, Indigo, Blue, Green, Yellow, Orange, and Red. The visible light is a part of the electromagnetic spectrum. The electromagnetic spectrum consists of a range of frequency ranging from a few Hertz to 1025 Hertz.
Complete step-by-step solution:
The human eye can view lights only of a certain wavelength, one of which being the visible light part of the electromagnetic spectrum whose wavelength ranges between 380×10−9mto750×10−9m.
We will see the wavelength ranges of different colours of the electromagnetic spectrum:
- The wavelength of violet light ranges from 380×10−9m to 450×10−9m.
- The wavelength of blue light ranges from 450×10−9m to 495×10−9m.
- The wavelength of green light is 495×10−9m to 570×10−9m.
- The wavelength of yellow is 570×10−9m to 590×10−9m.
- The wavelength of orange light is 590×10−9m to 620×10−9m.
- The wavelength of red light is 620×10−9m to 750×10−9m.
When we look at the above options, blue light has wavelength ranging from 4500A0−4950A0
Hence, option D is the correct answer.
Note: The electromagnetic spectrum all together is a band consisting of Gamma radiation, X-ray radiation, Ultraviolet radiation, visible light, infrared radiation, Microwave radiation, Radio waves.
In the electromagnetic wave, a lower wavelength or higher frequency wave will have more energy. Therefore, gamma rays have the most energy and radio waves having the least energy. Electromagnetic waves interact with matter via their electric and magnetic fields which set in oscillation charges present in all matter. The detailed interaction and so the mechanism of absorption, scattering, etc., depend on the wavelength of the electromagnetic wave, and the nature of the atoms and molecules in the medium.
