Question
Question: What is the activation energy for a reaction if its rate doubles when the temperature is raised from...
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20°C to 35°C? (R=8.314Jmol−1K−1log2=0.3010)
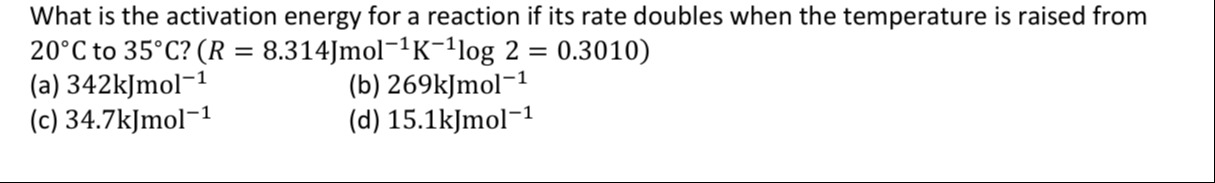
342kJmol−1
269kJmol−1
34.7kJmol−1
15.1kJmol−1
The correct answer is 34.7kJmol−1
Solution
The relationship between the rate constant (k) and temperature (T) is given by the Arrhenius equation. For two different temperatures T1 and T2, the rate constants k1 and k2 are related by the integrated Arrhenius equation:
ln(k1k2)=REa(T11−T21)=REa(T1T2T2−T1)where Ea is the activation energy and R is the ideal gas constant.
Given:
T1=20∘C T2=35∘C
The rate doubles when the temperature is raised from 20°C to 35°C, which means k2=2k1, so k1k2=2.
R=8.314 Jmol−1K−1 log2=0.3010
First, convert the temperatures from Celsius to Kelvin:
T1=20+273.15=293.15 K T2=35+273.15=308.15 K
Now, calculate ln(k1k2):
ln(k1k2)=ln(2)
Using the relationship ln(x)=2.303log10(x):
ln(2)=2.303×log10(2)=2.303×0.3010=0.693023
Now, calculate the temperature difference and the product of temperatures:
T2−T1=308.15−293.15=15 K T1T2=293.15×308.15=90400.9725 K2
Substitute these values into the integrated Arrhenius equation:
0.693023=8.314 Jmol−1K−1Ea(90400.9725 K215 K) 0.693023=8.314Ea×90400.972515Solve for Ea:
Ea=0.693023×8.314×1590400.9725 Ea=0.693023×8.314×6026.7315 Ea≈5.7603×6026.7315 Ea≈34724.8 J/molTo convert the activation energy from joules per mole to kilojoules per mole, divide by 1000:
Ea≈100034724.8 kJ/mol Ea≈34.7248 kJ/molThis value is closest to 34.7 kJ/mol.
