Question
Question: What is the action of the alcoholic solution of potassium hydroxide on ethyl bromide?...
What is the action of the alcoholic solution of potassium hydroxide on ethyl bromide?
Solution
The alcoholic potassium hydroxide is used for the formation of the alkene from alkyl halide. Alkyl halide undergoes an elimination reaction with alcoholic potassium hydroxide to give an alkene. The alcoholic potassium hydroxide acts as a base.
Complete step by step answer:
When alcoholic solution of potassium hydroxide reacts with alkyl halide, the alkyl halide undergoes elimination reaction. The hydrogen and halogen get eliminated and alkene is formed.
The reaction of action of the alcoholic solution of potassium hydroxide on ethyl bromide is as follows:
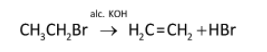
When an alcoholic solution of potassium hydroxide reacts with ethyl bromide, ethyl bromide undergoes an elimination reaction. The hydrogen and bromine get eliminated and ethene is formed.The alcoholic potassium hydroxide acts as a base so it gives hydroxide ion which abstracts a proton from beta-carbon of ethyl bromide and forms a carbanion.
This beta-carbanion attacks on alpha-carbon to form a double bond and the bromide ion is removed. So, the product ethene forms.
Therefore, the action of the alcoholic solution of potassium hydroxide on ethyl bromide forms ethene as a product and during this action hydrogen bromide removes.
Note:
The reaction is also known as dehydrohalogenation because hydrogen and halogen are removed from the alkyl halide. The reaction is a beta-elimination because hydrogen present at beta-carbon is removed during the reaction. The action of alcoholic potassium hydroxide is different from aqueous potassium hydroxide. The action of alcoholic potassium hydroxide gives ethene whereas the action of aqueous potassium hydroxide on ethyl bromide gives ethanol.
