Question
Question: What is the action of hot hydroiodic acid on isopropyl methyl ether?...
What is the action of hot hydroiodic acid on isopropyl methyl ether?
Solution
The carbon-oxygen bond in ethers can be cleaved by heating with the halogen acid such as hydroiodic acid HI . The general reaction of the ether with the hydroiodic acid is as shown below,
R−O−R (Ether) +HX(Halogen acid)373 KROH(Alcohol)+RX(Alkyl halide)
Complete Solution :
Ethers are the least reactive of the functional groups. Carbon-oxygen bond in ethers can be cleaved under drastic conditions.
Hot hydroiodic acid reacts with the isopropyl methyl ether. This results in the cleavage of the carbon-oxygen bonds. Thus reaction of isopropyl methyl ether hot hydroiodic acid gives Propan-2- ol, and iodomethane or methyl iodide.
The cleavage of the reaction of isopropyl methyl ether follows the following mechanism:
1. The ether molecule being base gets protonated ether or oxonium salt.
The reaction takes place in the presence of hydroiodic acid because these reagents are sufficiently acidic.
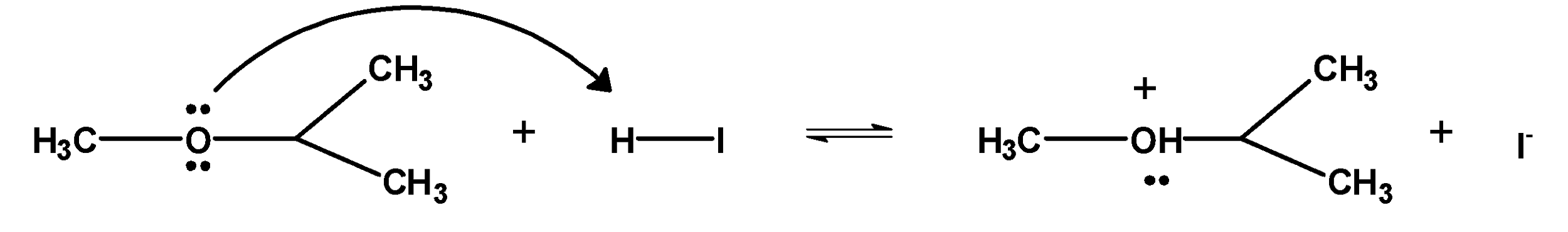
2. Iodide ion I− is a good nucleophile. The protonated ether undergoes nucleophilic attack by iodide ion and displace an alcohol molecule by SN2 mechanism and therefore, forms alkyl alcohol and alkyl halide.
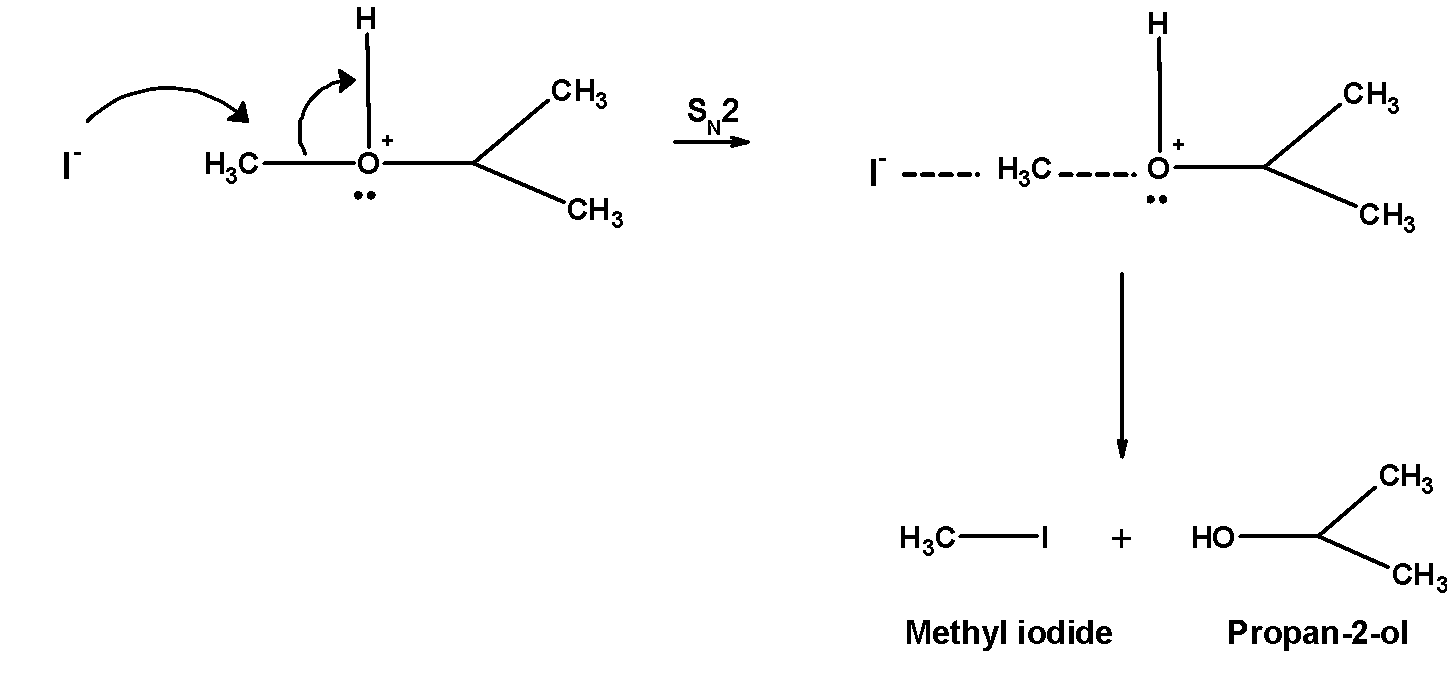
Remember that, in the case of unsymmetrical ethers with two different alkyl groups, the size of the cleavage is such that the halide is formed from the alkyl group which is smaller in size. The reaction proceeds via SN2 the mechanism. The SN2 mechanism favours well with primary carbon atom .f primary and secondary alkyl groups are present then halide attack on the less hindered alkyl group.
Thus reaction of isopropyl methyl ether hot hydroiodic acid gives Propan-2- ol, and iodomethane or methyl iodide.
Note: When HI is in excess and the reaction is carried out at a high temperature, methanol formed reacts with another molecule of HI and is converted into methyl iodide.
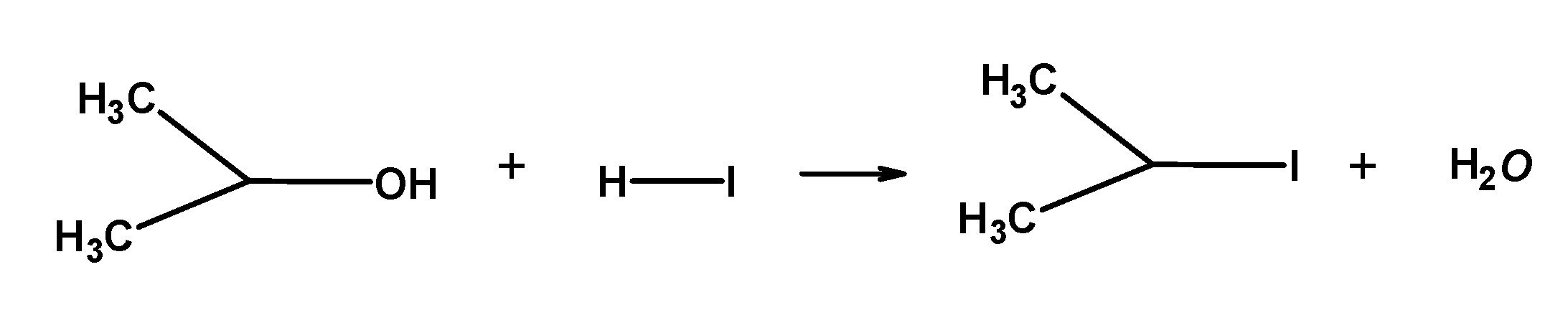
The order of reactivity of halogen acid is: HI > HBr > HCl
