Question
Question: What is the action of ammonia \(N{{H}_{3}}\) on benzoic acid? Write equations....
What is the action of ammonia NH3 on benzoic acid? Write equations.
Solution
Benzoic acid has carboxyl group and ammonia is a base, so acid donates Hydrogen ion and ammonia accepts hydrogen ion to form ammonium salt and on heating high temperature, amide is formed.
Complete step by step answer:
- The simplest aromatic carboxylic acid is benzoic acid. Benzoic acid is naturally present in many plants.
- Molar mass of benzoic acid is 122.
- Benzoic acid is prepared by oxidation of Toluene. On hydrolysis of Benzonitrile and Benzamide leads to formation of Benzoic acid.
- Benzyl alcohol on oxidation produces Benzoic acid.
- Benzoic acid is meta directing, so substituent is added to meta position to carboxyl group.
-benzoic acid is a weak acid and ammonia is a weak base, acid and base react to form salt and water.
-Benzoic acid is a weak acid, so it donates proton to ammonia and ammonia is a weak base, it accepts proton to form salt Ammonium Benzoate.
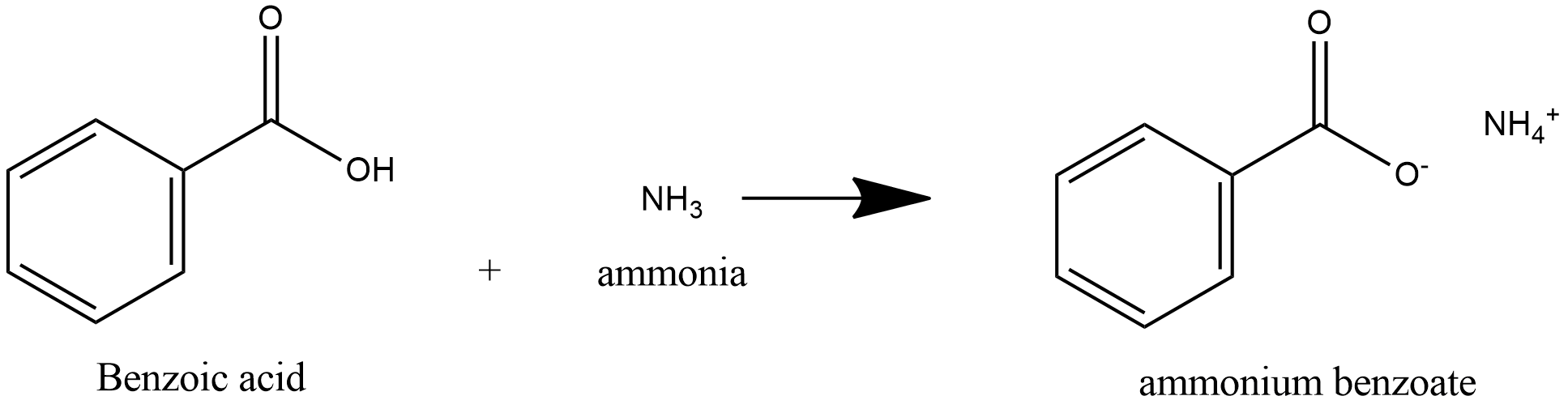
Further heating of ammonium benzoate leads to loss of water and leads to formation of Benzamide.
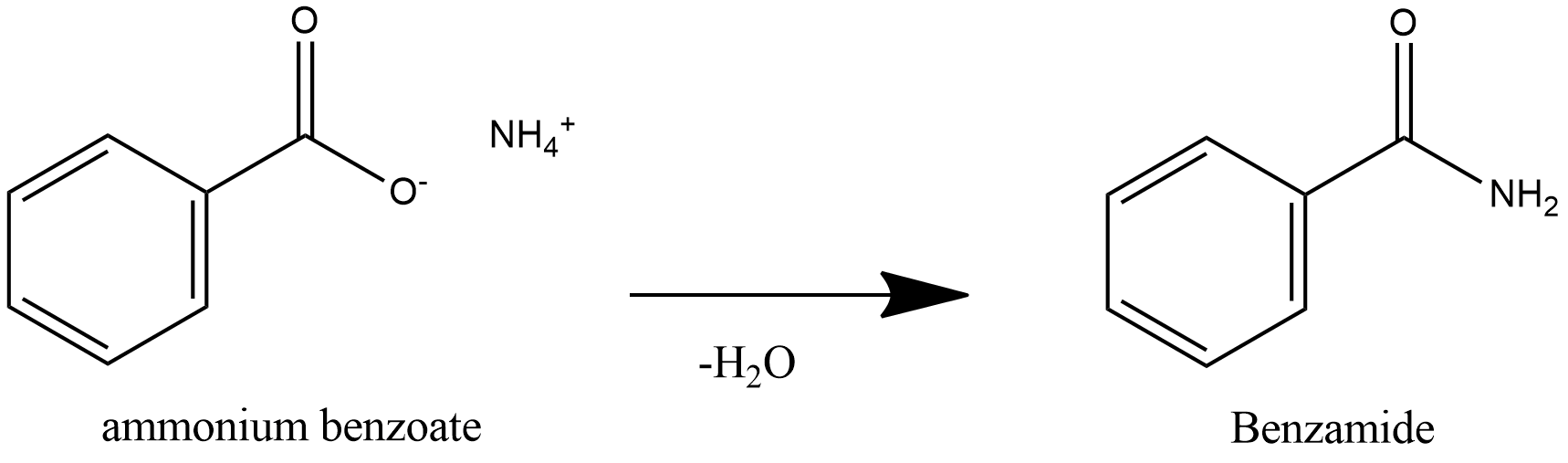
Benzamide on Hydrolysis produces Benzoic acid and Ammonia.
By carboxylation of phenyl magnesium halide, Benzoic acid is produced.
On vigorous oxidation of Alkyl benzene, benzoic acid is produced.
Ester, anhydride and acyl chloride on hydrolysis produces Carboxylic acid.
So, Benzoic acid reacts with ammonia to produce salt ammonium benzoate which on heating produces Benzamide.
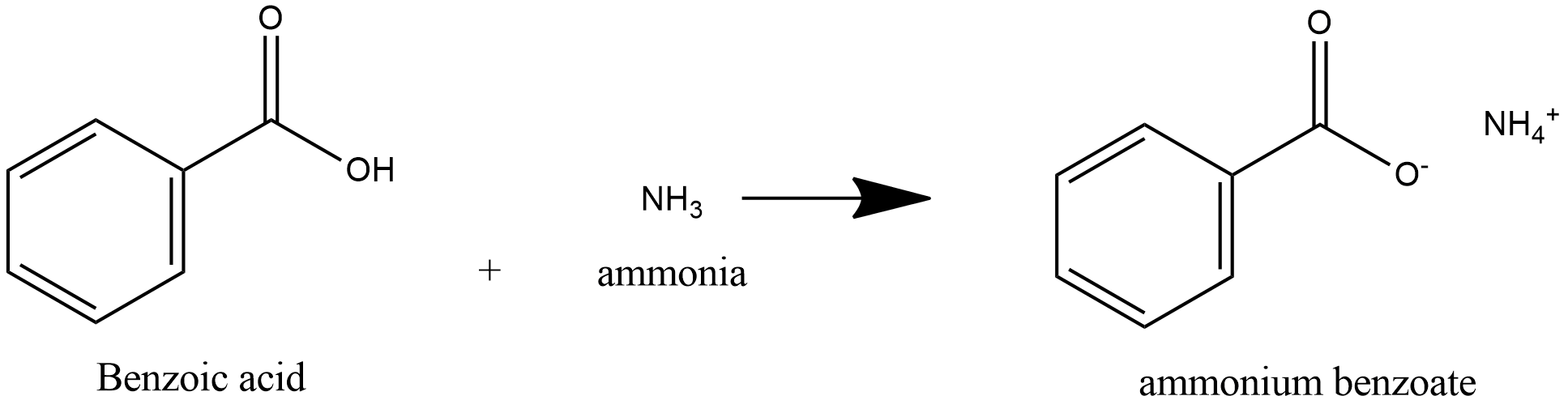
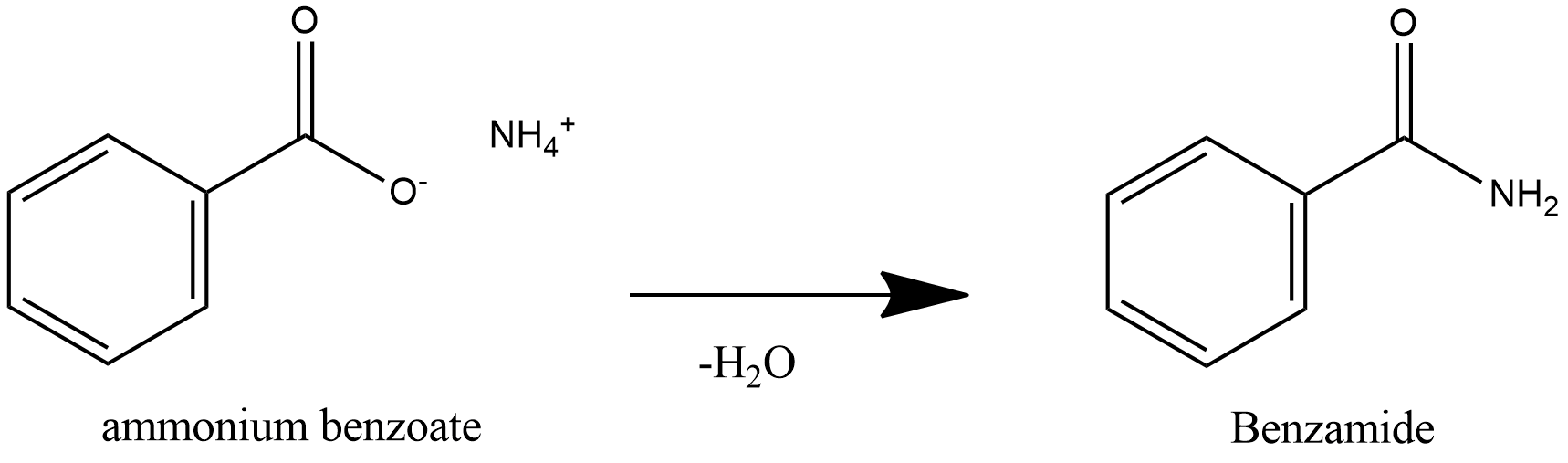
Note: Benzoic acid is a weak acid and ammonia is weak base, acid and base react to form salt and water. Benzoic acid is a weak acid, so it donates proton to ammonia and ammonia is a weak base, it accepts proton to form salt Ammonium Benzoate. Further heating of ammonium benzoate leads to loss of water and leads to formation of Benzamide.
