Question
Question: What is the acidity order of the following compounds? 
(a)- I > II > III > IV > V
(b)- II > III > IV > V > I
(c)- V > III > I > II > IV
(d)- V > III > II > I > IV
Solution
If the benzoic acid has an electron-donating group, then the stability of the carboxylate ion will decrease, so the acidity will decrease. If the benzoic acid has an electron-withdrawing group, then the stability of the carboxylate ion will increase, so the acidity will increase. But the compound is present on the ortho position, then its acidity will be the highest regardless of the nature of the substituent.
Complete step by step answer: If the benzoic acid has an electron-donating group, then the stability of the carboxylate ion will decrease, so the acidity will decrease. If the benzoic acid has an electron-withdrawing group, then the stability of the carboxylate ion will increase, so the acidity will increase. But the compound is present on the ortho position, then its acidity will be the highest regardless of the nature of the substituent, this is known as ortho-effect.
So, in the compounds given above, there are two compounds in which the methyl group is present at the ortho position, i.e., V and III. Since V has two methyl groups at the ortho position, it will be the highest acidic compound. After that, the III will be the most acidic. Now, we know that a methyl group is an electron-donating group, therefore, II and IV are less acidic than I.
The acidic due to methyl group on the benzoic acid is due to the hyperconjugation effect, the hyperconjugation effect at the p-position has left effect than the meta position, therefore, II will be more acidic than IV.
The order will be V > III > I > II > IV.
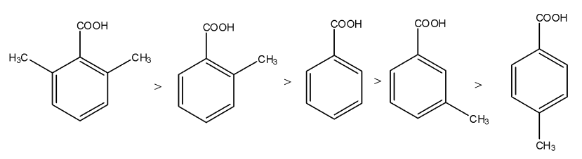
Therefore, the correct answer is an option (c).
Note: If the compounds are not at the ortho position, then the compounds like NO2, Cl, etc will increase the acidity, and compounds like CH3, OH, OCH3, etc will decrease the acidity.
