Question
Question: What is sublimation?...
What is sublimation?
Solution
In sublimation the substance does not pass through the liquid state they directly move from the solid to gaseous state. Sublimation process happens when the particles of a solid absorb enough energy to overcome the forces of attraction between them.
Complete answer:
Sublimation is just another phase transition for the process changes in phases of solids, liquids, and gases. As a sublimating substance directly changes from a solid to a gas, it never passes through the liquid state. The below image represents water in its three forms: ice, water, and steam. Sublimation is therefore, just one of the ways that water or another substance can change between its potential phases.
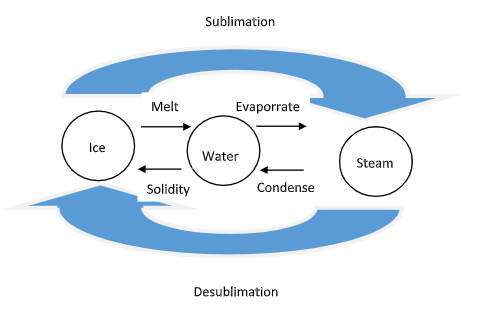
At the common and typical atmospheric pressure, we know that water is a solid at temperatures below 0 degrees Celsius, a liquid from 0 to 100 degrees Celsius, and a gas at higher temperatures. Atmospheric pressure, however, can change, particularly with altitude. Therefore, higher altitudes yield lower atmospheric pressures.
Note: Sublimation refers to the transition of a substance directly from the solid to the gaseous state., without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is referred to as deposition or desublimation, in which a substance passes directly from a gas to a solid phase.
