Question
Question: What is Stephen’s reaction?...
What is Stephen’s reaction?
Solution
To answer this question, you should recall the concept of Stephen Reaction. It involves the formation of aldehydes from cyanides in the presence of tin chloride.
Complete step by step answer:
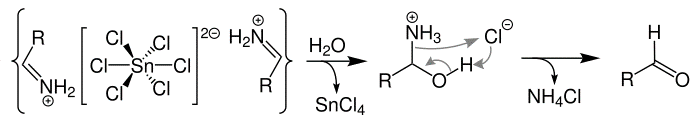
Stephens reaction is used in the preparation of aldehydes from nitriles using tin(II) chloride and hydrochloric acid in presence of water .
The mechanism of this reaction can be summarized as:
Step 1: Addition of gaseous hydrogen chloride to nitrile. The reaction can be represented using the below equation and forming a salt:
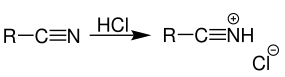
Step 2: Transfer of electron from tin(II) chloride which reduces the previously formed salt. The reaction can be represented using the below equation:
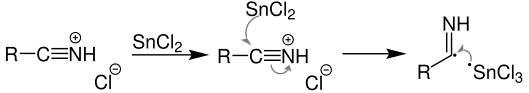
This formed salt reacts with hydrochloric acid resulting in:
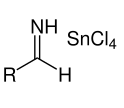
Step 3: The salt formed with hydrochloric acid precipitates shortly. The reaction can be represented using the below equation:
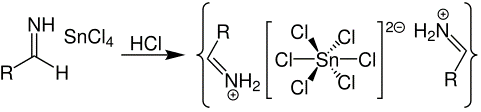
Step 4: The salt formed is aldimine chloride. Hydrolysis of this salt results in the formation of an amide. The reaction can be represented using the below equation which results in the formation of an aldehyde:
Note:
Another important reaction of aldehydes is aldol condensation. The reaction of aldehydes and ketones containing at least one α hydrogen is treated with dilute alkali; they form β -hydroxy aldehydes or β -hydroxy ketones (ketol) respectively. We know that in aldol condensation the hydroxide ion functions as a base moving the acidic hydrogen-producing the reactive enolate ion. Further, the aldehyde is attacked at the electrophilic carbonyl carbon by the nucleophilic enolate ion resulting in an alkoxide intermediate. The alkoxide ion now formed deprotonates the water molecule, ultimately resulting in hydroxide and the β –hydroxy aldehyde.
