Question
Question: What is \(s{p^2}\) hybridized carbon atom?...
What is sp2 hybridized carbon atom?
Solution
Intermixing of orbitals of slightly different energies in order to redistribute their energies resulting in the formation of new sets of orbitals of equivalent energies and shape. This process of intermixing is called hybridization while the new orbitals formed are called hybrid or hybridized orbitals.
Complete answer:
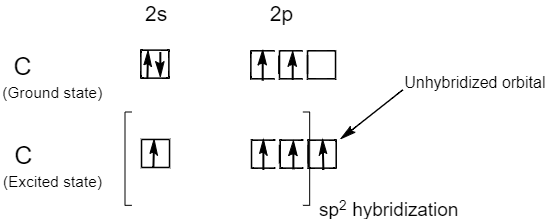
One s and two p orbitals hybridize to form three equivalent sp2 hybrid orbital. In each of the three sp2 hybrid orbital one third i.e.33% s character and two third i.e. 66% p character is present. The three sp2 hybrid orbitals lie in the same plane making 120∘ angle with each other. Therefore, molecules involving sp2 hybridization have a bond angle of 120∘ and trigonal planar geometry.
The carbon atoms of alkenes or carbon atoms bonded by double bonds i.e. C=C bond involves sp2 hybridization, because the atomic number of carbon is six and its ground state electronic configuration is 1s22s22p2 . But in an excited state a carbon atom has four unpaired electrons. Therefore, the one 2s orbital and two 2p orbital i.e. three orbital in total gets hybridized to form three sp2 hybrid orbital while one 2pz orbital remains unhybridized. Hence, carbon atoms in alkenes or carbon atoms bound by double bonds are sp2 hybridized carbon atoms.
Note:
A class of hydrocarbons which is unsaturated and contains at least one carbon to carbon double bond are called alkenes. Alkenes are planar molecules with sp2 hybridization and 120∘ bond angle, whereas alkynes are also a class of unsaturated hydrocarbon, containing at least one carbon to carbon triple bond. But, alkynes have linear geometry with sp hybridization and 180∘ bond angle.
