Question
Question: What is residual entropy?...
What is residual entropy?
Solution
The law states that entropy of a pure crystalline substance at absolute zero is zero. It does not have any practical application. As the temperature of crystals is increased from Zero Kelvin, the orderliness of the crystals are changed and due to the vibrations produced, it results in entropy.
Complete step by step answer:
-We already know what the third law of thermodynamics is. The law states that entropy of a pure crystalline substance at absolute zero is zero.
-A perfect crystal means that all the molecules are in the lattice arranged regularly.
-Pure crystalline substance means that all the atoms are arranged or aligned in the same direction as a perfect crystal. According to the third law, we know that entropy is zero in this case. But we know that it is not practically possible to have a crystal with no disorder.
-As the temperature is increased, the disorderliness increases and entropy is greater than zero.
-At absolute zero (zero Kelvin), the entropy is not zero and has a finite value for a molecule such as H2O, N2O etc.
These entropies are called the residual entropy.
-Residual entropy can be calculated as the difference between the experimental value of entropy and theoretical value.
-Let us now look at some of the examples:-
-Let us calculate the entropy of CO
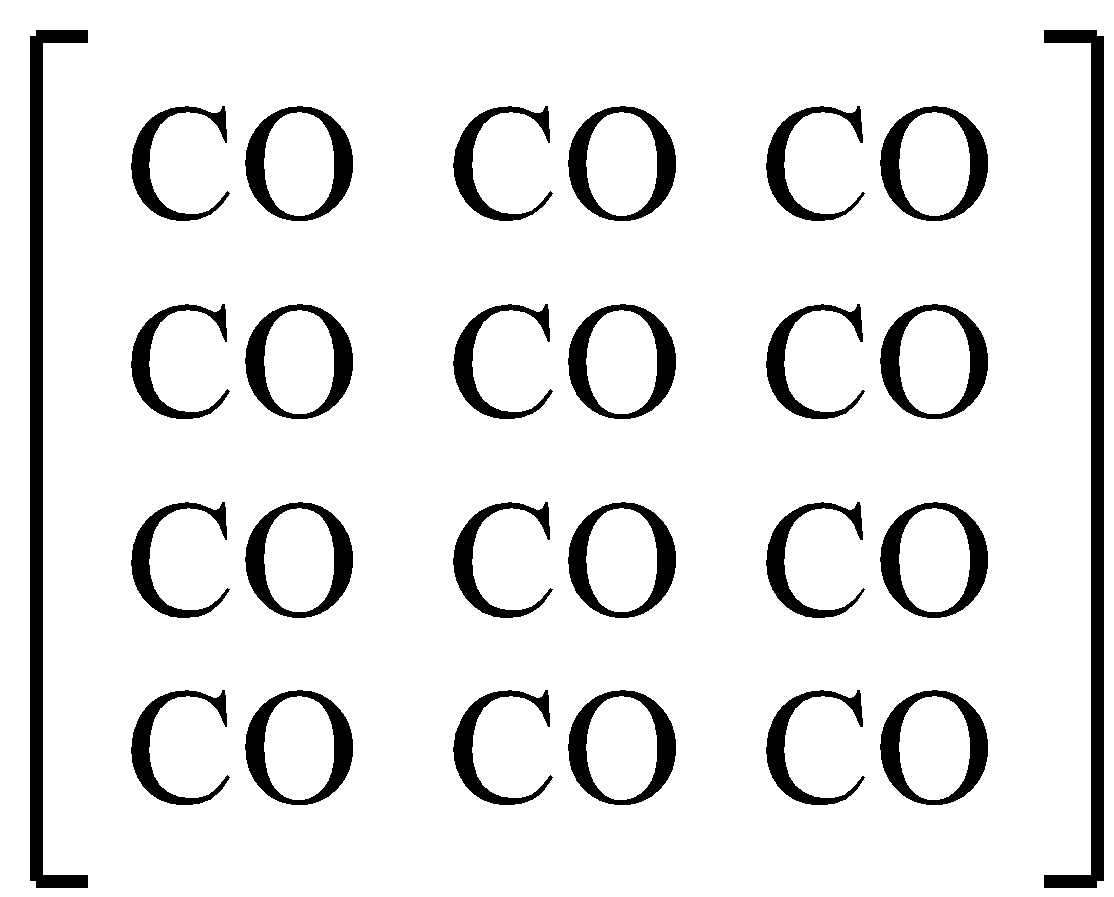
This is a perfect crystal- pure crystal
-A real crystalline form is given below with disorder.
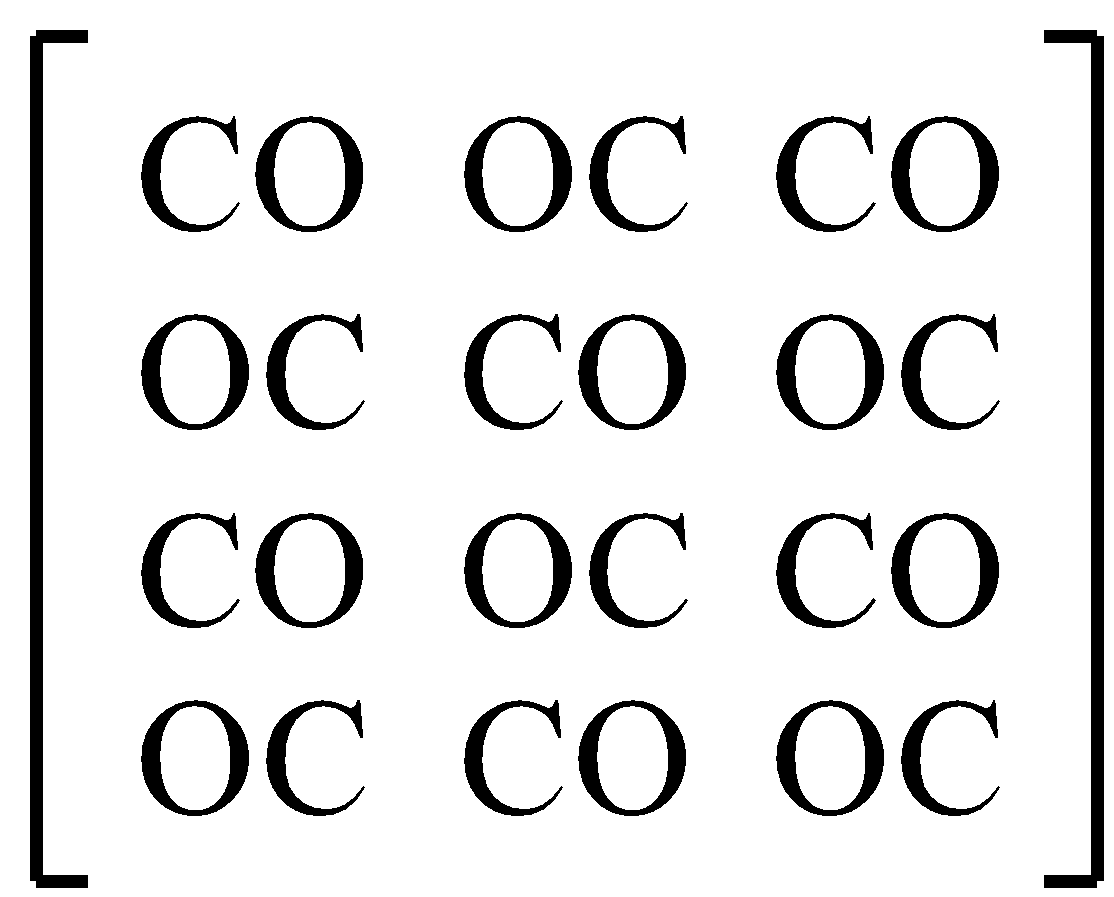
-We know that in one crystal, there is Avogadro’s number of molecules. Here, N numbers of CO molecules are present. (N is the Avogadro’s number).
-In this example, there are 2 orientations possible for one CO molecule - CO and OC.
-Therefore there can be 2N ways of having this possible orientation.
-W = 2N Where W is the thermodynamic probability.
-According to Boltzmann Entropy equation,
S = klnW
S = kln2N
S = Rln2N
-So, residual entropy is defined.
Note:
The value of residual entropy differs for different crystals according to the value of W, which is thermal probability. It depends on the maximum number of ways in which each molecule inside a crystal can be arranged in a different orientation. The third law has no practical application as the entropy cannot be zero. There is no possibility of a perfect crystal.
