Question
Question: What is primary ionization?...
What is primary ionization?
Solution
We need to know that during ionization there is gaining or removing of the electron and there is a formation of ions. The ionization energy is the energy required to remove an electron from the isolated gaseous atom. The ionization mainly depends on the size of an atom. The tendency to remove the electron of an atom increases with an increase in the size of the atom due to a decrease in the atomic radius and effective nuclear charge.
Complete answer:
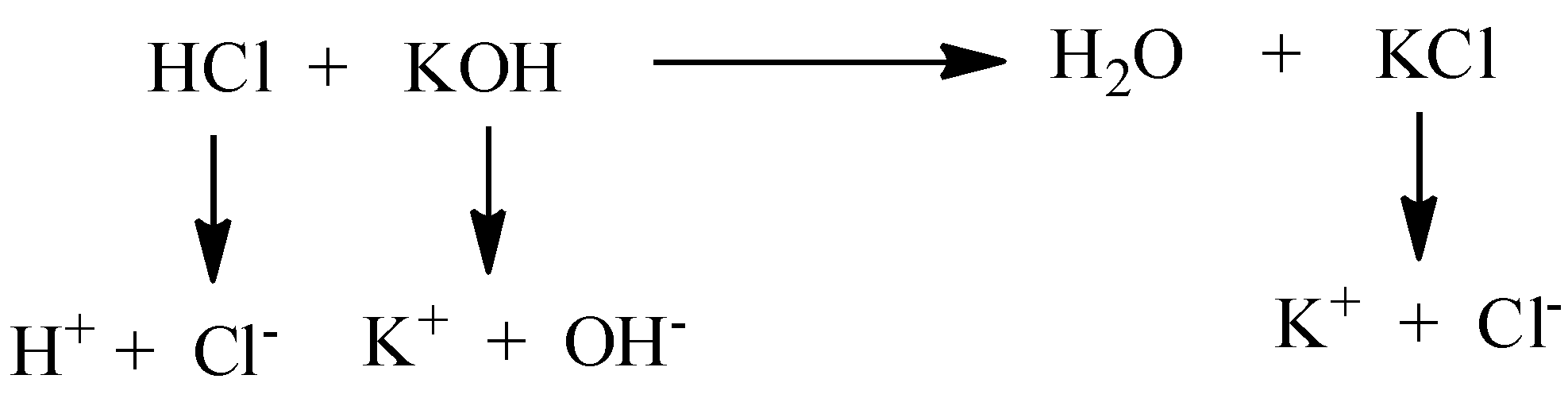
We need to know that ionization is the process by which the removal or gaining of an electron from the molecule or atom and there is a formation of ions. If a molecule or atom absorbs the electron, it produces a negative charge and it is known as anion. If the molecule or atom loses the electron, it produces a positive charge and it is known as cation. The ionization accompanied by passing particles is known as primary ionization.
The primary electrons are the electrons generated by primary ionization products. The primary ionization energy is always less than secondary ionization energy. Because, in the secondary stage, the size of the molecule will decrease and you want to apply more energy to remove the electron. Let’s see the example of ionization,
Note:
We must have to know that in the ionization process, there is a formation of anion as well as cation by the gaining and removing of electrons respectively from the atom. Consider one example, the chlorine atom is ionized by gaining one electron and it forms a negatively charged chlorine atom. Therefore, the ionization is the process of conversion of a normal atom to the ion.
