Question
Question: What is not true regarding the products? 
A. Product-I and II are position isomers
B. Product-I and II contains the same number of sp3 and sp2 carbon atoms
C. The yield of the product I and product II is same
D. Reaction obeys Saytzeff rule
Solution
We have to know that, the drying out response of alcohols to produce alkene continues by warming the alcohols within the sight of a solid corrosive, like sulfuric or phosphoric corrosive, at high temperatures.
Complete answer:
The given alcohol compound is butan−2−ol . This butan−2−ol reacts with concentrated hydrochloric acid to give product (I) alkene and product (II) alkene.
At the point when you get dried out a liquor, you eliminate the - OH bunch, and a hydrogen iota from the following carbon particle in the chain. With atoms like butan−2−ol , there are two prospects when that occurs.
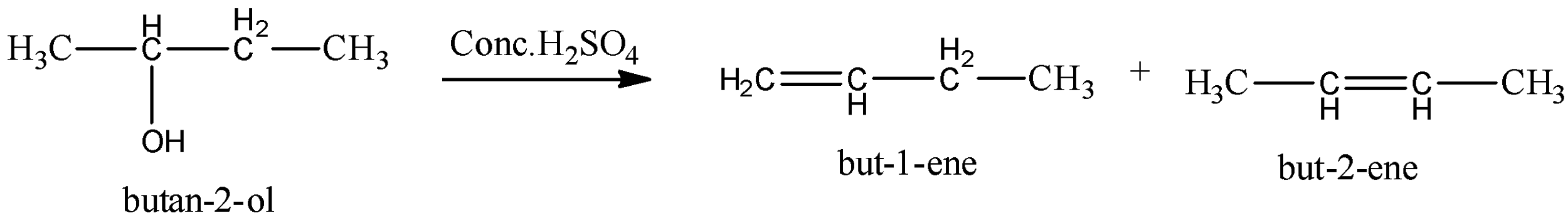
Drying out of butan-2-ol prompts a blend containing:
but−1−ene
cis−but−2−ene (otherwise called (Z)−but−2−ene )
trans−but−2−ene (otherwise called (E)−but−2−ene ).
Alcohols go through lack of hydration (loss of water particle) in acidic medium to give olefins. A twofold bond is shaped because of the loss of water particles. It's anything but an end-response. As indicated by Saytzeff's standard (likewise Zaitsev's standard), during lack of hydration, more subbed alkene (olefin) is shaped as a significant item, since more prominent the replacement of twofold bond more noteworthy is the dependability of alkene.
Therefore, option (C) is not true.
Note:
Consider the longest chain containing the twofold bond: If two gatherings (connected to the carbons of the twofold bond) are on a similar side of the twofold bond, the isomer is a cis alkene. On the off chance that the two gatherings lie on inverse sides of the twofold bond, the isomer is a trans alkene.
