Question
Question: What is meant by voids in crystal structure?...
What is meant by voids in crystal structure?
Solution
We know that Packing of atoms or molecules in crystals several possible arrangements but the one in which maximum available space is occupied.
Complete answer:
We can classify the close packing arrangements into square packing and hexagonal packing.
In the hexagonal arrangement, occupied space is 60.4% and the remaining 39.6% called voids.
In square packing, arrangement of occupied space is 52.4% and remaining 47.6% called voids.
We have to remember that the arrangement of a solid sphere with close packing which remains some space is called voids.
-In three dimensions it shows interstitial sites in close packing of the sphere. Consider a triangular or trigonal site. Also of the above sites shows two different sites.
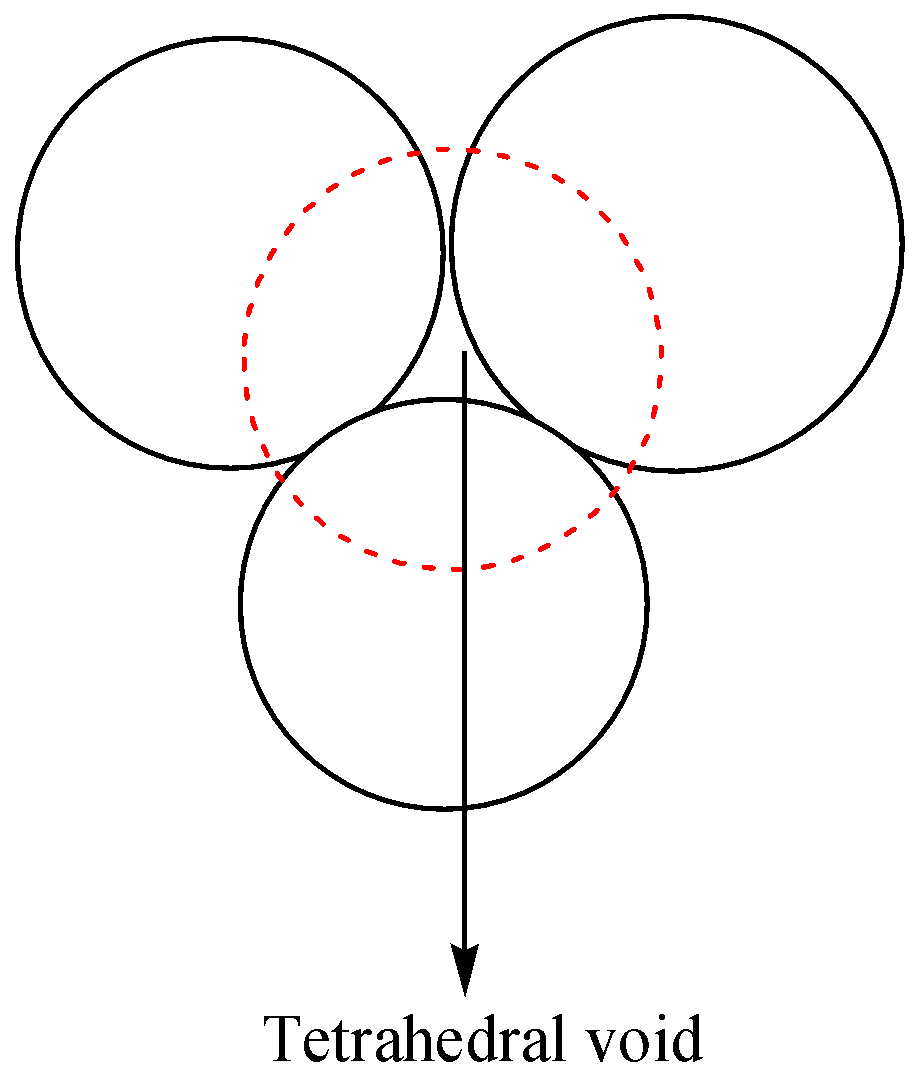
Now we discuss about the tetrahedral voids as,
-Tetrahedral sites: if three spheres are placed on the one spheres which are touching each other. Even four spheres touch one another some gaps available that know as the tetrahedral sites. In these sites, one sphere is surrounded by eight tetrahedral sites, and one site surrounded by four spheres. The coordination number is four and the ratio is 2:1.
The diagram representation of tetrahedral voids is given as,
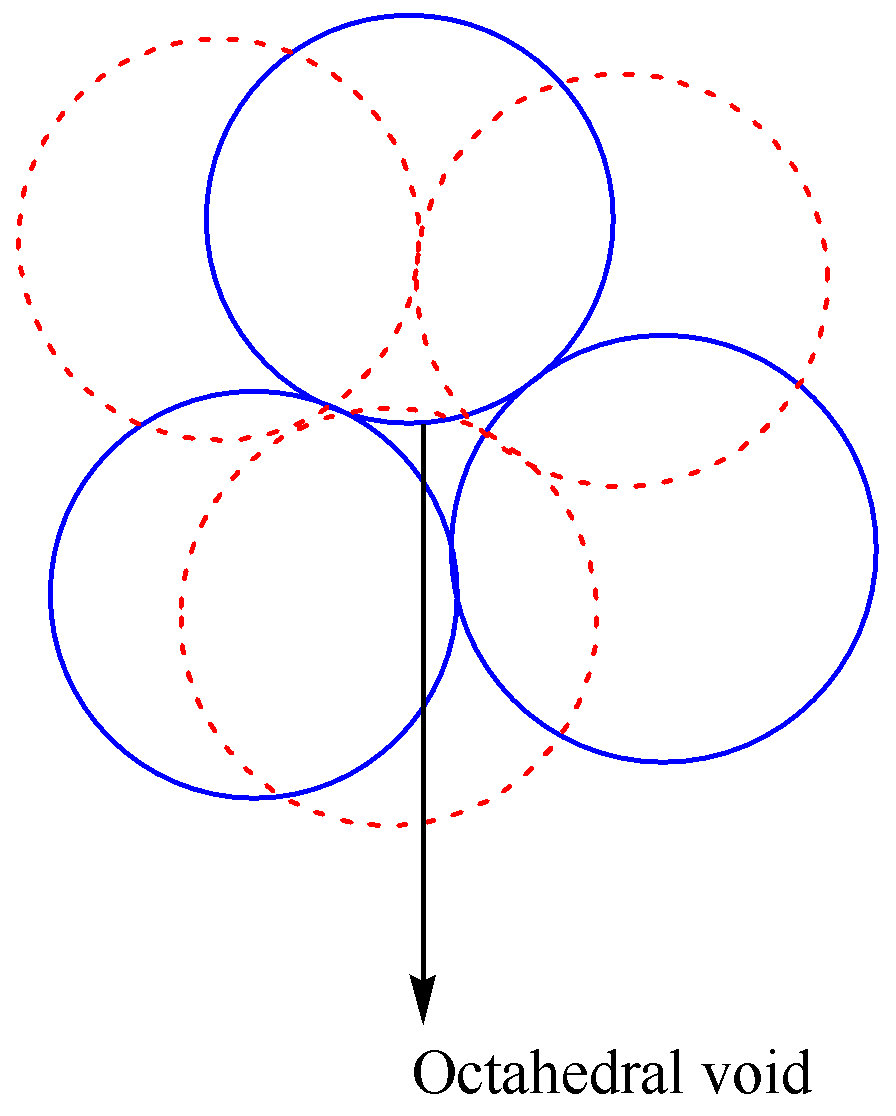
Now we discuss about the octahedral voids as,
Octahedral sites: The close packing of a sphere in hcp, as well as ccp arrangement, is known as octahedral sites. This shows six spheres attached in two forms. In these sites, one sphere is surrounded by six octahedral sites, and one site surrounded by six spheres. Hence the coordination number is six. Ratio is 1:1.
The diagram representation of octahedral voids is given as,
Note:
-We must remember that the voids in solid states mean the vacant between the atoms in a closely packed structure.
-Even in the closest packing of a sphere, there is some space left in between the spheres called interstitial sites.
