Question
Question: What is meant by the term chemical formula?...
What is meant by the term chemical formula?
Solution
We should recall the definition of a chemical formula. It is not a chemical name and it contains no words. It contains chemical symbols. It is a condensed form and can imply a fully structural formula.
Complete answer:
-As we know chemistry subjects are made of chemical formulas. Without chemical formulas, it is hard to define chemistry. We use chemical formula as a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas, plus (+) and minus (−) signs.
-For information, we should know that there are different types of chemical formulas and each type gives us different information about a chemical substance. The different types of chemical formulas include molecular, empirical, structural and condensed structural formulas. We'll discuss each of these different types as well as go over a few examples.
-In chemistry, we use different types of chemical formulae like empirical formula and molecular formulae. Such as if we take the example of glucose. Glucose empirical formula is CH2O (twice as many hydrogen atoms as carbon and oxygen), while its molecular formula is C6H12O6 (12 hydrogen atoms, six carbon and oxygen atoms).
| Molecular formula | Empirical formula | Structural formula |
|---|---|---|
| C6H12O6 | CH3 | 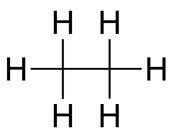 |
| C2H5OH | C2H6O | 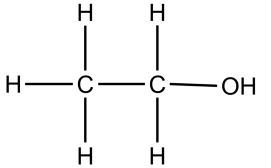 |
| CH3COOH | C2H4O2 | 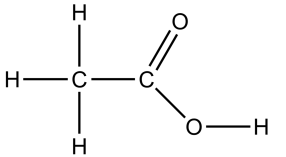 |
Additional Information:
-We know that chemical formulae may be used in chemical equations to describe chemical reactions and other chemical transformations, such as the dissolving of ionic compounds into solution. We should understand this thing that chemical formula is not a chemical name, and it contains no words.
Note:
To write a chemical formula, it is important to know that we should know about the symbol of the elements present in the compound, formula of the radicals and the valency of the elements in that compound. Most of the compounds that we find in chemistry are binary, that is they have two elements. As we know that an atom with a positive charge is called a cation whereas an atom with the negative charge is called an anion. Anions having -1 negative charge usually have a suffix as –ide. For example, (F−)Fluoride. Anions (oxygen + another element) usually have a suffix as “ate”. For example, (SO42−) Sulphate. When a polyatomic anion has H−ion, bi- or hydrogen is used as a suffix. For example, HCO3− bicarbonate or hydrogen carbonate.
