Question
Question: What is meant by refining of metals? Describe the electrolytic refining of copper with a neat labell...
What is meant by refining of metals? Describe the electrolytic refining of copper with a neat labelled diagram.
Solution
As we know that in chemistry, periodic tables play a vital role. Now we are using a modern periodic table. This modern periodic table consists of totally 118 elements. In the periodic table there are totally 18 columns and 7 rows. The columns are called groups. Hence, 18 groups in the periodic table. The rows are called periods. Hence, totally 7 period in the table. The atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons. In general we move left to right metallic property of the atom decreases. We move right to left and non-metallic property decreases.
Complete answer:
The refining of metal is given below,
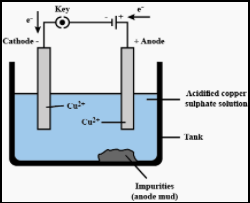
In electrolysis, the anode is made up of the impure metal and the cathode is made up of pure metal. The electrolytic solution is nothing but the solution of metal salt. When electric current is passing in an electrolytic solution, pure metal dissolves and settles at the cathode. The impurities settle down at anode and that is called anode mud.
The refining of copper is given below,
At anode: Cu→Cu2++2e−
At cathode: Cu2++2e−→Cu
The diagrammatic representation of electrolytic refining of copper is given below,
Note:
As we know, in the world there are a lot of elements in various forms. For study and developing purposes, classification happens. Periodic table used to study the elements in easy form. If one new element is discovered, it means, based on the physical and chemical property we placed in the periodic table. In the modern periodic table metallic atoms are placed in the left side of the table and non-metallic atoms are placed in the right side of the table. Metalloids and transition elements are placed in between metal and non-metal elements. Below the two rows are there. There are nothing but lanthanides and actinides. These are f-block elements.
