Question
Question: What is meant by refining metals? Describe the electrolytic refining of copper with a neat labeled d...
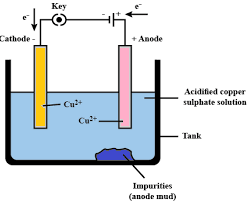
What is meant by refining metals? Describe the electrolytic refining of copper with a neat labeled diagram.
Solution
We know Electrolytic refining is a procedure that is utilized for the extraction and filtration of metals that are gotten by refining techniques. The sullied metal is utilized as an anode and the unadulterated metal is utilized as a cathode. Solvent salt from a similar metal is utilized as an electrolyte. At the point when an electric flow is passed, unadulterated metal gets saved at the cathode and the sullied metal gets broken down from the anode. Pollutions from the metal get gathered underneath the anode and are known as anode mud.
In the electrolytic refining contaminations like gold, silver, platinum bunch metals, arsenic, selenium, and tellurium are recuperated.
Complete answer:
In electrolytic refining measure, the debased metal is made as anode and a flimsy segment of unadulterated metal is made as cathode. An answer of the metal salt is made as an electrolyte. On going the current through the electrolyte, the unadulterated metal from the anode disintegrates into the electrolyte. A comparable measure of unadulterated metal from the electrolyte is stored on the cathode.
The dissolvable pollutions go into the arrangement, though, the insoluble debasements settle down at the lower part of the anode and are known as anode mud.
A square of debased copper is taken as an anode or positive cathode. Copper sulfate which is fermented with sulphuric corrosive is utilized as a graphite-covered electrolyte alongside unadulterated copper tubes, as a cathode or negative anode. In this period of electrolysis copper sulfate partitions into a positive particle of copper Cu2+ and a negative particle of sulfate. The positive copper particle Cu2+or cations travel towards the negative anode made of unadulterated copper where it retains the electrons from the cathode. Cu molecule is kept on the cathode's graphite layer.
The cathode is covered with graphite during the time spent electrolytic metal handling or just electro granulating so the concentrated material can be effectively eliminated. This is quite possibly the most developing electrolysis system.
Anode reaction: CuCu2++2e−
Cathode reaction: Cu2++2e−Cu
Note:
In present day times, the principle utilization of copper is as an electrical transmitter. Copper has a high for every unit volume electrical conductivity. It very well may be effectively drawn through strings, regardless of whether single or multi fiber, which can be helpfully and consistently curved without excessive durability of activity. Copper wire is promptly tinned, has better patching properties and is safer than consumption at resources.
