Question
Question: What is meant by positive and negative deviation from Raoult’s law and how is the sign of \({\Delta ...
What is meant by positive and negative deviation from Raoult’s law and how is the sign of ΔmixH related to positive and negative deviations from Raoult’s law?
Solution
We know Raoult’s law. The vapor pressure of a solution containing non-volatile solute is proportional to the mole fraction of the solvent at a given temperature.
The mathematical expression of Raoult’s law is,
Psolution=xsolventP∘solvent
Complete step by step answer:
We must know that the non-ideal solution expresses positive and negative deviations from their ideal behaviour.
Now, we see Positive deviation.
Also positive deviation occurs if the total pressure of the solution is more than the resultant vapor pressure.
Now, consider a binary solution containing two components A and B. If the interaction between A and B are weaker than the interaction between A-A and B-B then the escape tendency of A and B molecules from the solution becomes more than from pure liquids therefore the partial pressure of each component is greater. This is termed as positive deviation.
P=PA+PB>P∘AXA+P∘BXB
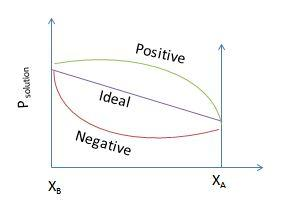
The sign of ΔmixH is positive since energy is needed to break the attractive forces between A-A and B-B and it is an endothermic process.
Let us see negative deviation.
Negative deviation occurs if the total pressure of the solution is less than the resultant vapor pressure.
Now, consider a binary solution containing two components A and B. If the interaction between A and B are stronger than the interaction between A-A and B-B then the escape tendency of A and B molecules from the solution becomes less than from pure liquids therefore the partial pressure of each component is lower. This is termed as negative deviation.
P=PA+PB<P∘AXA+P∘BXB
∴ The sign of ΔmixH is negative since energy is released due to increase in the attractive forces between A-A and B-B and it is an exothermic process.
Note: We must know that Raoult's law has some imitations. Therefore,
Raoult's law is only suitable to describe the ideal solutions.
The liquids in the mixture do not have uniform attractive forces so these types of solutions do not obey Raoult's law.
