Question
Question: What is meant by hydroboration-oxidation reaction? Illustrate it with an example....
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Solution
Hydroboration oxidation is an anti-markovnikov addition of water across alkene. Also, it is a two-step organic reaction. End product is alcohol.
Complete step by step solution:
- It is known to you that hydroboration oxidation is a two-step organic reaction.
- It is an organic reaction that converts an alkene into an alcohol by the net addition of water across the double bond. THF(Tetrahydrofuran) is used as a solvent.
- The hydrogen (H) and hydroxyl group (OH) are added in a syn (addition of addendum on the same side of the alkene or alkyne) addition leading to cis stereochemistry.
- Hydroboration-oxidation is an anti-Markovnikov’s addition of water across an alkene. The -OH group is added to the less substituted position and the -H is in the more substituted position.
- If there is more than one chiral center in the product, then the product will produce a pair of enantiomers that results from syn addition. A common example of oxidation of 1-Propene is given below.
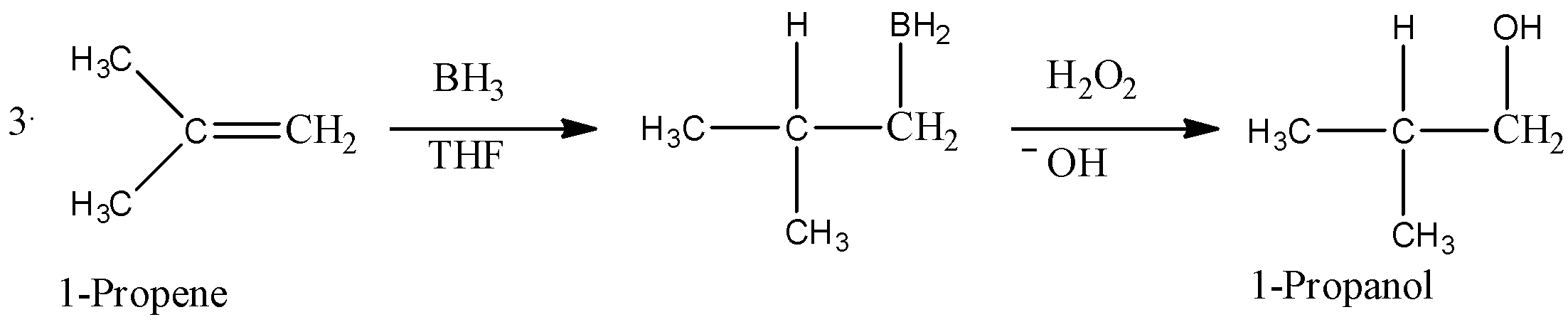
- Here, we can see that first, BH3 is added to the carbon-carbon double bond in a way that H-atom gets bonded to a carbon that has less number of hydrogen atoms(means in anti-markovnikov manner). Then, in presence of hydrogen peroxide-an oxidising agent, the organo-borane compound decomposes and gives an alcohol as shown in the reaction.
- One molecule of BH3 can oxidise three molecules of alkene because one B-H bond is responsible for the oxidation of one molecule of alkene. After oxidising alkene, B-H bond gets converted into B-OH bond. So, BH3 has three B-H bonds, and it will oxidise total of three alkenes and will give H3BO3 in the end. We can show how one molecule of BH3 oxidises three molecules of alkene as below.
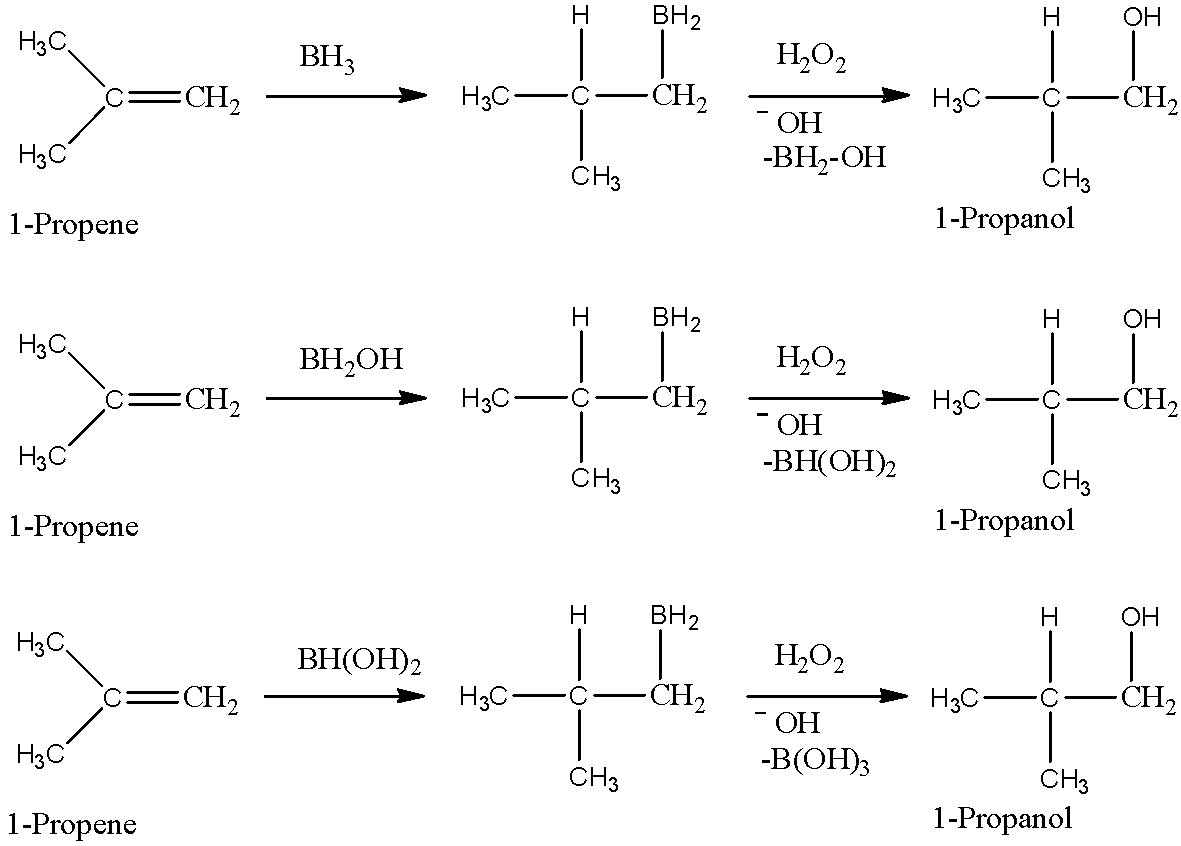
Note: Remember that it will give an anti-markovnikov product after addition to the alkene double bond. We cannot use water as a solvent here because it will immediately react with BH3 as water has an amphoteric nature and BH3 is a lewis acid.
