Question
Chemistry Question on Hybridisation
What is meant by hybridisation of atomic orbitals? Describe the shapes of sp,sp2,sp3 hybrid orbitals.
Hybridization is defined as an intermixing of a set of atomic orbitals of slightly different energies, thereby forming a new set of orbitals having equivalent energies and shapes.
For example, one 2s−orbital hybridizes with two 2p−orbitals of carbon to form three new sp2 hybrid orbitals.
These hybrid orbitals have minimum repulsion between their electron pairs and thus, are more stable. Hybridization helps indicate the geometry of the molecule.
Shape of sp hybrid orbitals: sp hybrid orbitals have a linear shape. They are formed by the intermixing of s and porbitals as:
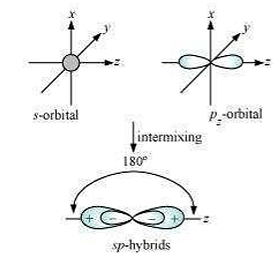
Shape of sp2 hybrid orbitals:
sp2 hybrid orbitals are formed as a result of the intermixing of one s−orbital and two 2p−orbitals. The hybrid orbitals are oriented in a trigonal planar arrangement as:

Shape of sp3 hybrid orbitals:
Four sp3 hybrid orbitals are formed by intermixing one s−orbital with three p−orbitals. The four sp3 hybrid orbitals are arranged in the form of a tetrahedron as:

