Question
Question: What is general formual of below mentioned silicate? [o : oxygen; ⚫ : silicon] ...
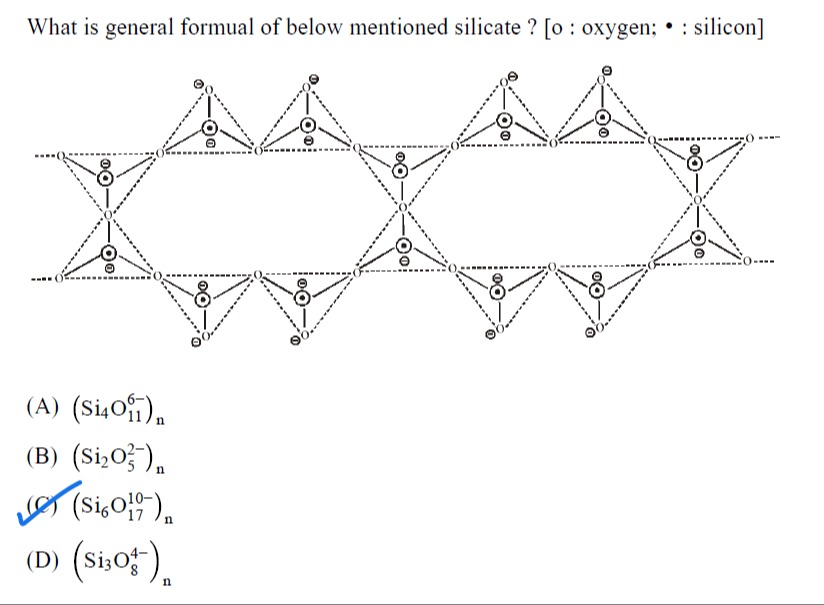
What is general formual of below mentioned silicate? [o : oxygen; ⚫ : silicon]
A
(Si4O116−)n
B
(Si2O52−)n
C
(Si6O1710−)n
D
(Si3O84−)n
Answer
(Si4O116−)n
Explanation
Solution
The given structure is a double chain silicate. The general formula of a double chain silicate, like amphibole, is (Si4O116−)n. Each silicon atom is tetrahedrally coordinated with oxygen atoms. The repeating unit consists of linked tetrahedra forming a chain-like structure. By counting the silicon and oxygen atoms in the repeating unit, and considering the charge balance, the general formula can be determined.
