Question
Question: What is entropy? Write its units....
What is entropy? Write its units.
Solution
This is thermodynamic function. Generally, it is used to express the extent of disorder in a system.
Complete step by step answer:
Entropy is a measure of randomness or disorder of a system. It is usually represented by ‘S’.
Let us explain entropy by giving example of states of matter i.e. gas, liquid and solid
As we know molecular arrangement of solid, liquid and gas are as follows
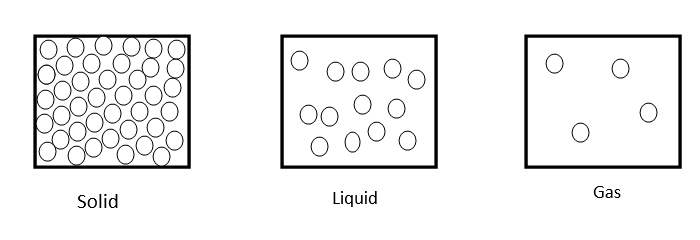
Molecular attraction in solid is greatest, in liquids it is moderate and in gaseous state it is least.
∴ Randomness of molecules in gas, liquid, solid decrease as
gas > liquid > Solid.
Since randomness in molecules of gas is greater due to least attraction and decreases from liquid and then solid due to increased attraction between molecules.
∴ Entropy of states of matter in the order
Gas > liquid > solid.
Entropy is a state function and entropy change during a process given by ΔS=S2−S1
WhereS2=∑Sproduct S1=∑Sreactant
Change in entropy of system changes as follows
(i) When the system absorbs energy in the form of heat, the kinetic energy of the system increases. As a result disorder vibration of molecules increases.
∴ entropy of the system also increases.
(ii) Entropy is inversely related to temperature
ΔS=Tqrev
Where, qrev= heat absorbed in reversible process.
T= Absolute temperature.
For same amount of heat at low temperature entropy i.e. randomness is more
∴ΔS is inversely proportional to absolute temp T
Unit of entropy is Cal k−1 mol−1 in C.G.S system and J K−1 mol−1 in S.I. system.
Note:
Entropy is extensive property. Entropy increases when solid melts as sublimes or decomposes to give gas or liquid. Entropy also increases when the number of molecules of a product is greater than the number of molecules of Reactant.
