Question
Question: What is enthalpy of formation of $NH_3$ if bond enthalpies are as $(N \equiv N) = 941$ kJ, $(H-H) = ...
What is enthalpy of formation of NH3 if bond enthalpies are as (N≡N)=941 kJ, (H−H)=436 kJ, (N−H)=389 kJ?
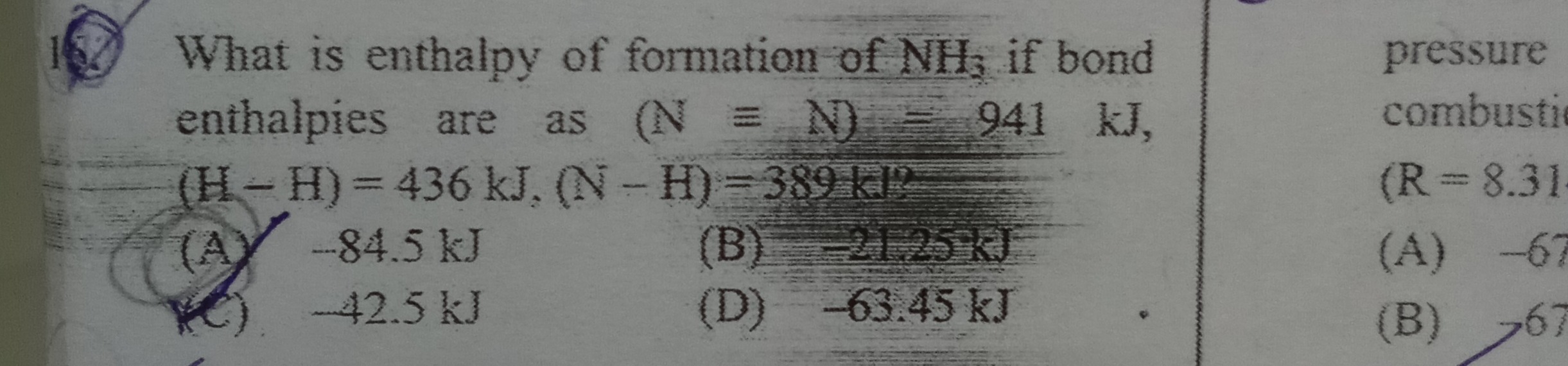
A
-84.5 kJ
B
-21.25 kJ
C
-42.5 kJ
D
-63.45 kJ
Answer
-42.5 kJ/mol
Explanation
Solution
For the formation of ammonia:
Reaction: N2+3H2→2NH3-
Energy to break bonds:
- Breaking 1 mole of N≡N: 941kJ
- Breaking 3 moles of H–H: 3×436=1308kJ
Total energy absorbed =941+1308=2249kJ.
-
Energy released forming bonds:
- Forming 6 moles of N–H bonds: 6×389=2334kJ
-
Net Reaction Enthalpy (ΔH):
ΔHrxn=2249−2334=−85kJSince this is for 2 moles of NH3, the enthalpy of formation per mole is:
ΔHf=2−85=−42.5kJ/mol
