Question
Question: What is Curtius Rearrangement?...
What is Curtius Rearrangement?
Solution
Curtius Rearrangement reaction is the preparation reaction of Amines from Acid Chloride as starting material. Hydrazine is used as the reagent in this particular rearrangement.
Complete step by step answer:
The curtius rearrangement is a reaction where acid chloride is converted to lower homologous amine.
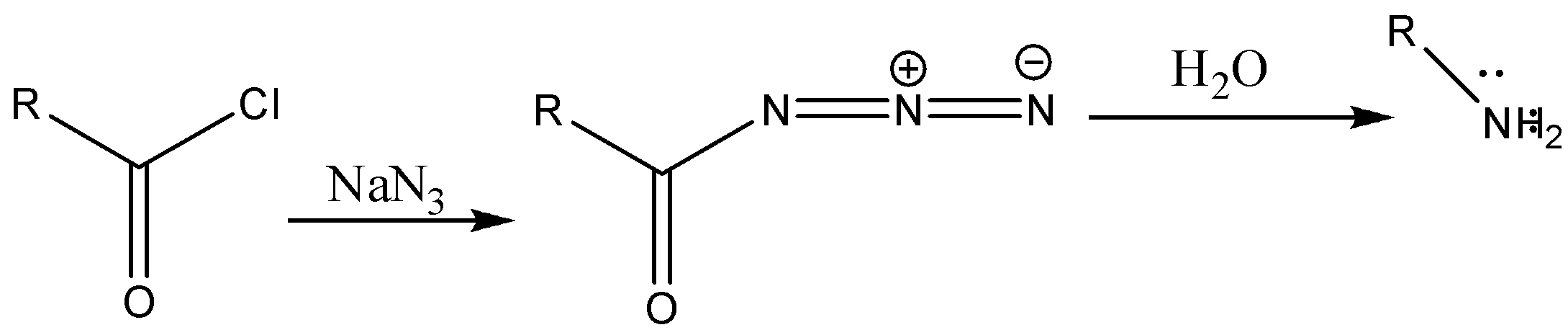
Reaction with mechanism is as follows:
Step 1-The starting material in the reaction is the acid chloride. The acid chloride on reaction with sodium azide will produce acid azide. The reaction will be as follows:
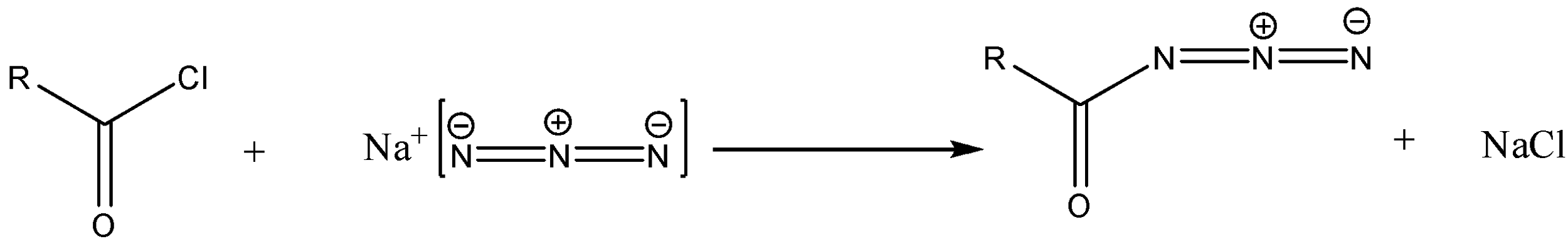
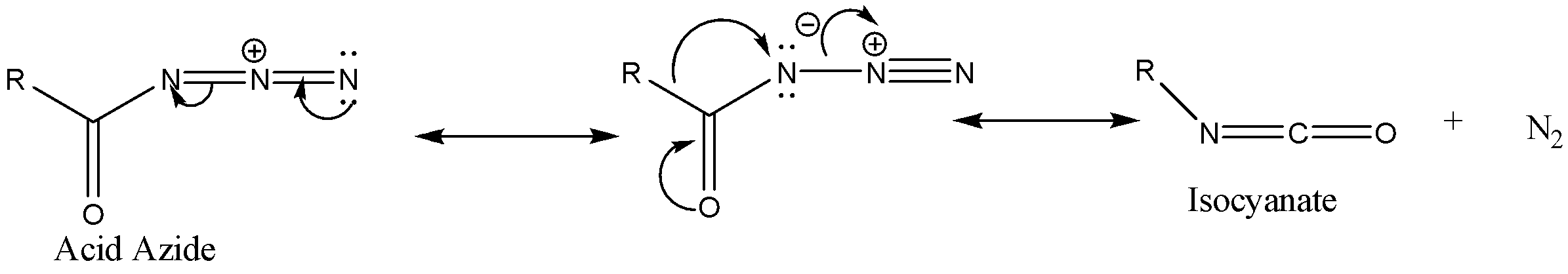
Step 2: The acid azide formed due to thermal decomposition then undergoes rearrangement thus releasing nitrogen gas and Isocyanate is formed.
Step 3: Isocyanate formed reacts in a different manner when different solvent is used. The reaction is carried out in aqueous or alcohol solution. Isocyanate gets converted to primary amine or urethane respectively.
Case 1: Reaction in Aqueous medium.
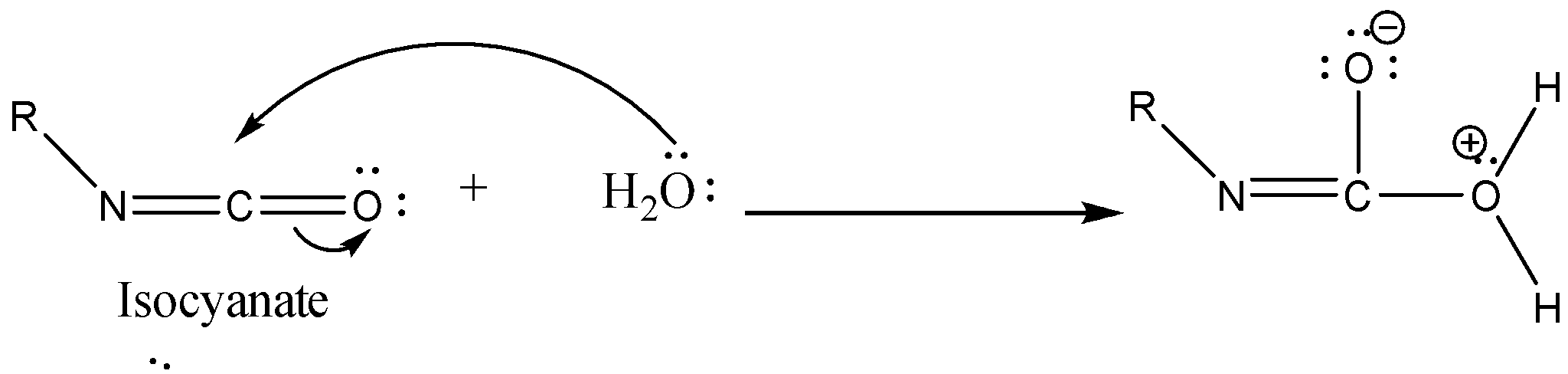
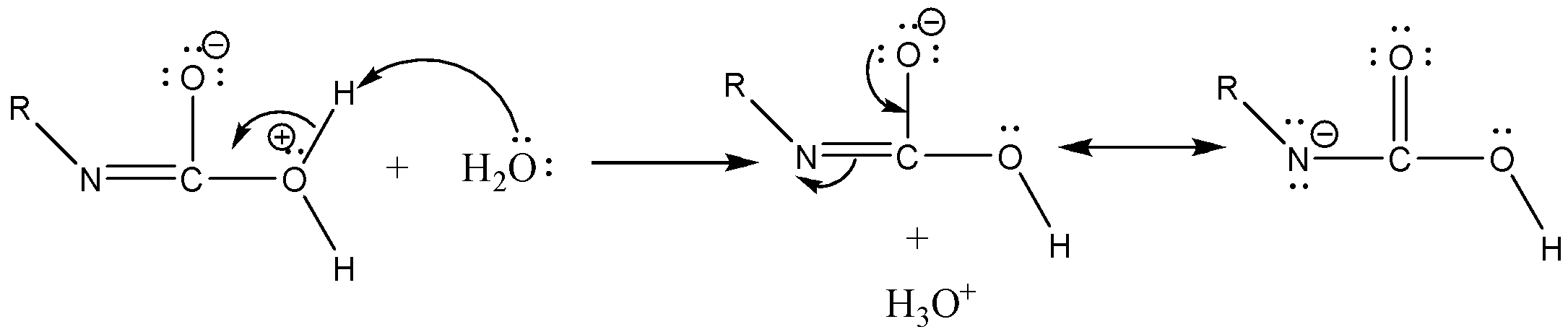
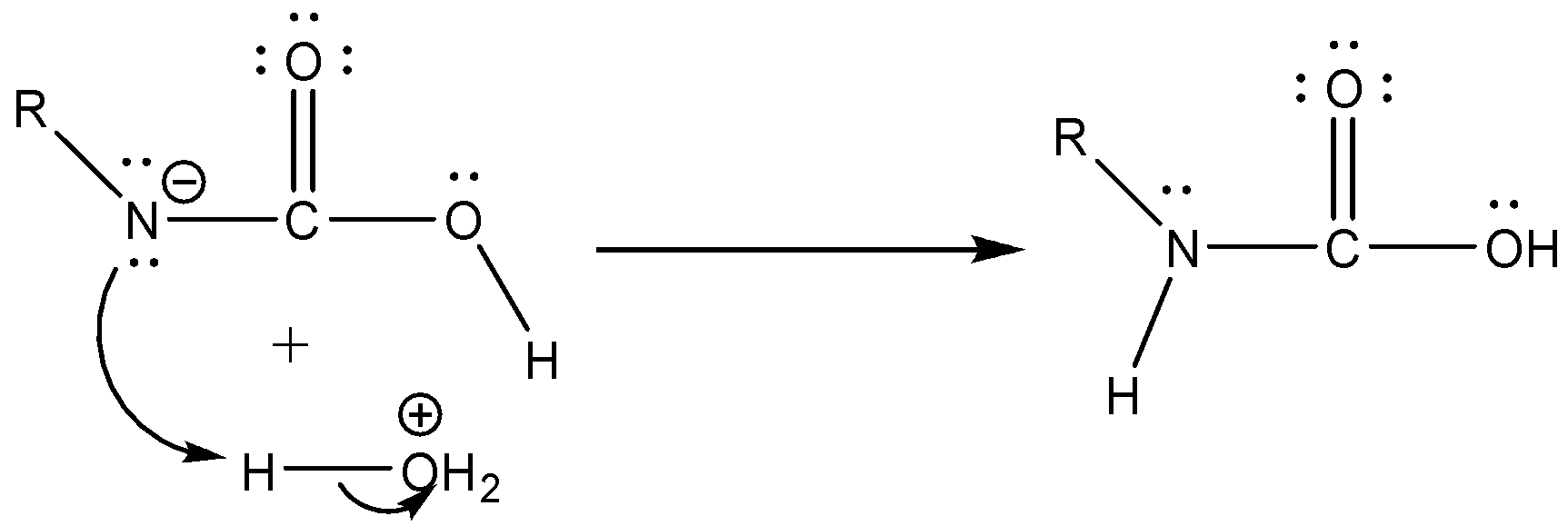
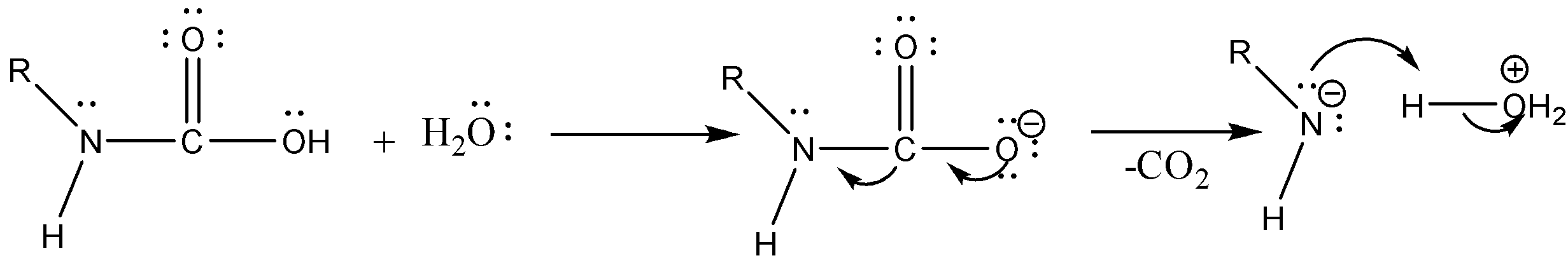
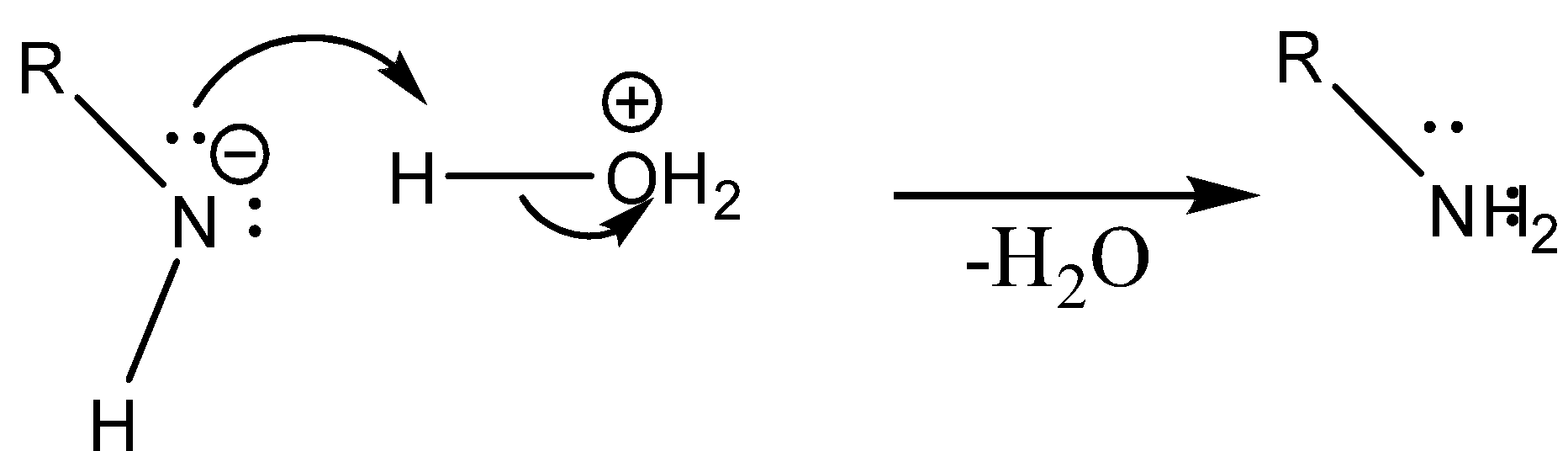
In this reaction the carbonyl group is removed due to which the number of carbon atoms in the product reduces by one. The amine group is then attached to the terminal carbon atom.
Case 2: Reaction in alcoholic solvent.
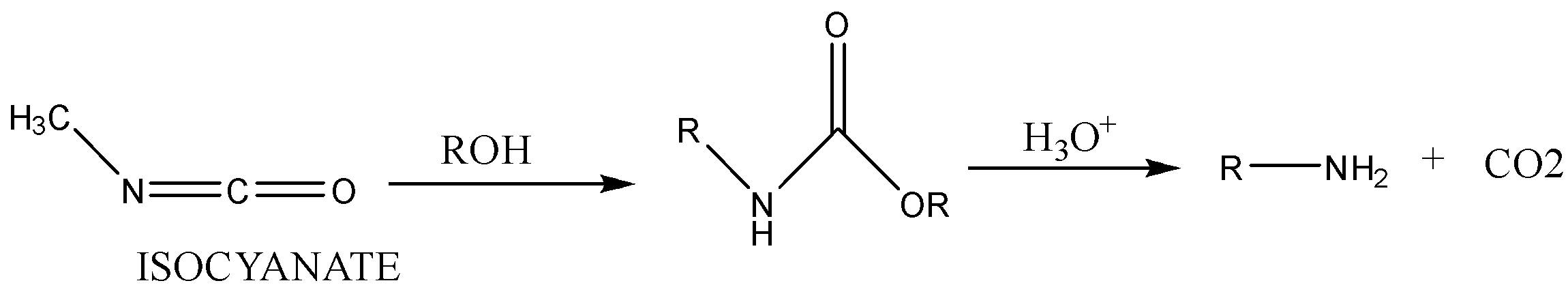
Note: This reaction involves a migration of alkyl or aryl groups from adjacent atom carbon atom to electron deficient nitrogen atom.
In this reaction the carbonyl group is removed due to which the number of carbon atoms in the product reduces by one. The amine group is then attached to the terminal carbon atom.
