Question
Question: What is Boyle temperature?...
What is Boyle temperature?
Solution
**Hint: ** Boyle temperature is described for real gases. Real gases do not adhere to the law of ideal gas and the particles of real gases have volumes.
**Complete step-by-step solution: **
Boyle temperature can be defined as the point in the temperature range in which a real gas starts to behave like an ideal gas at a pressure range. The temperature at which the second coefficient in the expression becomes zero is known as a Boyle temperature. This Boyle temperature balances out the attractive and the repulsive forces that are acting on a gas particle.
We can use the virial equation of state to calculate the Boyle temperature. Boyle temperature can be expressed in the terms of the virial coefficients.
Z=1+VmB+....
**Additional information: **
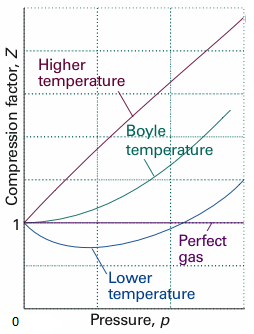
The above graph is plotted between the pressure pand compressibility factorZ. Boyle temperature is nicely marked in the graph. From this point, the real gas starts to behave like an ideal gas. The compression factor Z is given as,
Z=RTpVm
Here, Zis the compressibility factor, Vm is the volume,R is the gas constant, T is the
temperature and p is the pressure.
Z=1 for an ideal gas. Real gases show some deviation.
Note: Critical Temperature is different from Boyle's temperature. At the critical temperature, a gas shows non-ideal behavior. Critical temperature is lower than the Boyle temperature. At Boyle temperature a gas starts to behave like an ideal gas and for ideal gas, the compressibility factor is 1, that is, Z=1
