Question
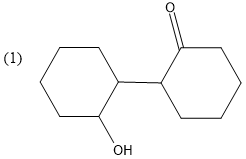
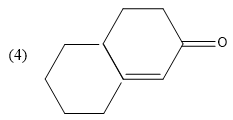
Question: What is B in the reaction below? 
Solution
We know that PCC stands for Pyridinium chlorochromate. It is used in organic chemistry as a reagent. It is used as a mild oxidising agent and can convert alcohols to carbonyls. KOH (Potassium hydroxide) is a base and can abstract acidic protons. So, based on the uses and functions of these reagents, we can find the products A and B in the above given reaction.
Complete answer:
In order to solve these types of questions in organic chemistry, we should first know about the reagents used in the reaction and their uses.
So, PCC stands for Pyridinium chlorochromate. It is used in organic chemistry as a reagent. It is used as a mild oxidising agent and can convert alcohols to carbonyls.
KOH (Potassium hydroxide) is a base and can abstract acidic protons. Also this reaction is taking place in the presence of heat. Heat leads to the dehydration reaction in which the water molecule is removed from the compound.
So, in first step the alcohol will get converted to ketone as it is a secondary alcohol as follows-
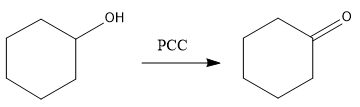
So, compound A is cyclohexanone.
In second step, when KOH reacts with cyclohexanone (carbonyl group), then aldol reaction will take place (two units of cyclohexanone will be consumed) and aldol will be formed as a product as follows-
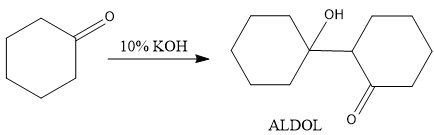
Now, heat is applied on this product and dehydration (removal of water molecule) will take place as follows-
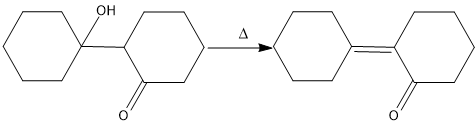
Hence, the correct option is
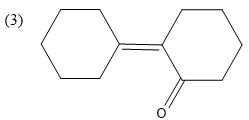
Note:
We should not get confused between the aldol condensation reaction and cannizzaro reaction because to proceed with aldol condensation there should be at least one alpha hydrogen and to proceed with the cannizzaro reaction there should be no alpha hydrogen. The reagents used for both these reactions are the same.
