Question
Question: What is an example of a tetrahedral bent molecule other than water?...
What is an example of a tetrahedral bent molecule other than water?
Solution
The shape or geometry of molecules is a result of the presence of bond pairs and lone pairs in that molecule. The lone pairs of electrons acquire a place in the molecule to minimize repulsion and therefore they distort the geometry of the molecule.
Complete answer:
Water has its chemical name as hydrogen oxide. It has 2 bond pairs and 2 lone pairs of electrons that gives it a tetrahedral bent shape. The lone pairs occupy such a position so that they can minimize repulsion, hence the geometry is tetrahedral.
Water molecule has oxygen as the central atom, similarly another molecule, like water, is H2S. As oxygen and sulphur both are from group 16, they tend to form hydrides with hydrogen with 2 bonds and 2 lone pairs of electrons. So, H2S molecule also has the same geometry as that of water molecule. The geometry of H2S is:
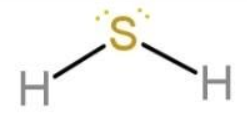
The difference is only in the bond lengths. Sulphur and hydrogen bond length is longer in H2S (133.6 pm), while oxygen and hydrogen bond length is shorter in H2O(95.8 pm). This is due to the fact that the size of atoms increases down the group, due to increase in the valence shells.
Hence, H2S molecule is a tetrahedral bent molecule other than water.
Note:
These geometries of molecules are according to the VSEPR. This theory suggests that the shape of any molecule depends on a bond and lone pair of electrons. The repulsive interactions are in the order, lone pair – lone pair repulsion > lone pair – bond pair repulsion > bond pair – bond pair repulsion.
