Question
Question: What is a primary, secondary and tertiary halide?...
What is a primary, secondary and tertiary halide?
Solution
Halogens are electronegative in nature and include fluorine, chlorine, bromine and iodine. Alkyl halides are those compounds in which one of the hydrogen atoms of the alkane is replaced with halide. Negative part of the compound is called halide.
Complete answer:
Alkyl halides are those compounds in which one of the hydrogen atoms of the alkane is replaced with halide. The general formula for alkyl halides is CnH2n+1X. Where n is a natural number. When n=1, then the formula becomes CH3X which is called methyl halide. Halides can be fluorides, chlorides, bromide and iodides. In order to form other members of primary halides, one of the hydrogen of methyl halide is replaced with a carbon atom. This is illustrated in the following diagram.
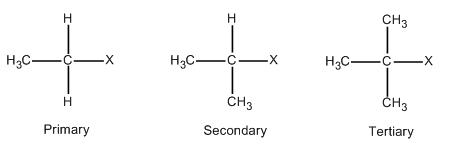
In case of secondary halides, two of the hydrogen of methyl halide is replaced with carbon atoms as described in the above diagram. On the similar lines, three of the hydrogen of methyl halide is replaced with carbon atoms in case of tertiary halides which is also described in the above diagram.
Additional Information: Due to electronegativity difference between halogen and carbon, C−Xbond is polar in nature where Xcarries a δ− negative charge while alkyl moiety carries a δ+charge.
Note:
It is important to note that methyl halide is the simplest primary halide. In order to form other members of primary halides, one of the hydrogen of methyl halide is replaced with a carbon atom. In case of secondary and tertiary halides, two and three of the hydrogen of methyl halides are replaced with carbon atoms, respectively.
