Question
Question: What is A in the following reaction: \(A\left[ {{C}_{6}}{{H}_{10}}{{O}_{3}} \right]\left( keto\tex...
What is A in the following reaction:
A[C6H10O3](keto ester)NaOH+I2Δyellow ppt+BH+CΔ−CO2CH3COOH
A.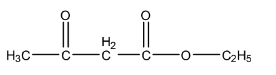
B.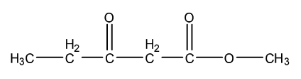
C. Both are Correct
D. None of correct
Solution
Keto esters are going to respond towards iodoform test and form respective sodium salts with the help of the chemicals sodium hydroxide and iodine at higher temperature. We can identify the keto esters by using iodoform test.
Complete step-by-step answer: - In the question there is a chemical equation which contains chemicals A, B and c and some chemicals on the arrows.
- With the help of the chemicals on the arrows we are supposed to find the structure of the chemical A.
- We can see that in the given chemical reaction there is a formation of acetic acid as the final product.
- By keeping all the chemicals on the arrows and the final product we can write the complete chemical reaction as follows.
A[C6H10O3]NaOH+I2Δyellow productCH3I+B
We got only CH3I as a product with B.
- So, the estimated structure of A will be as follows.
.
- Because if we consider the compound which is in option B we will get a different product on the iodoform test.
- The entire chemical reaction can be written as follows.
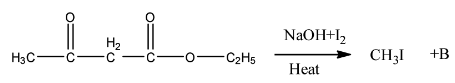
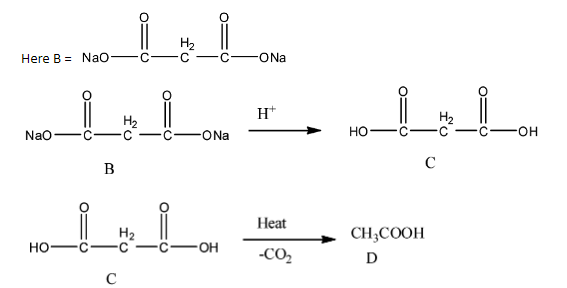
- Therefore the ketone ester after completion of the chemical reaction forms acetic acid (D) as the product.
- The overall reaction is going to occur if we took option A as the reactant.
So, the correct option is A.
Note: Iodoform test is very useful in the field of organic chemistry to find the presence of keto esters in the given compound. The keto esters have two functional groups, one is keto and the second one ester functional group.
