Question
Question: What is A in reaction below? 
What is A?
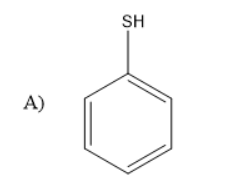
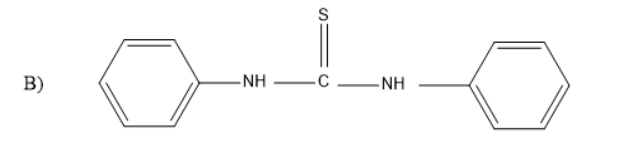
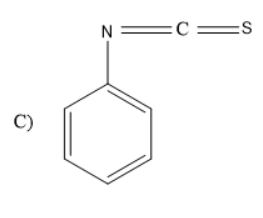
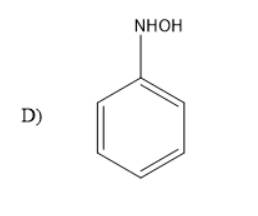
Solution
Aromatic compounds are the compounds that are cyclic, planar, conjugation of pi-electrons and obeying Huckel’s rule. Aniline is an aromatic compound and undergoes substitution reactions. Aniline is treated with carbon disulphide in presence of mercury salts to form phenyl isothiocyanate.
Complete answer:
Given compound is aniline. Aniline is an aromatic compound as it is cyclic, planar i.e., all the carbon atoms are sp2, conjugation of π electrons and the compound obey Huckel’s rule. The huckel’s rule is given by (4n+2)π electrons, where n is a whole number.
As the aniline consists of 6πelectrons satisfying Huckel's condition. It is an aromatic compound.
When aniline is treated with carbon disulphide in presence of mercury salts like HgCl2, the amine group in the aniline rearranges to form iso thiocyanate group. Thus, phenyl isothiocyanate will be formed.
This reaction can be one of the important named reactions in organic chemistry called the Hoffmann mustard oil reaction.
Thus, the given reaction is Hoffmann mustard oil reaction in which the formed products are isothiocyanates which have very unpleasant or pungent odour and are very dangerous as they consist of cyanides or nitriles. Thus, the product formed will be
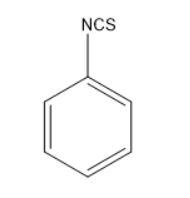
So, the correct answer is “Option C”.
Note:
Cyanides have the functional group as -CN, but the thio cyanate indicates the -NC, the iso thio cyanates have the functional group as -NCS. The iso thio cyanates have a very pungent odour and not be inhaled. So, precautions must be taken while dealing with this reaction.
