Question
Question: What is a benzylic position?...
What is a benzylic position?
Solution
Aromatic compounds are stable, and involved in resonance. Benzene is an aromatic compound, all the derivatives of benzene are also aromatic compounds. The positions in benzene will be given different names. The carbon attached directly to the benzene ring is termed as benzylic carbon.
Complete answer:
Benzene is an aromatic compound, aromaticity is the property exhibited by the molecules which are planar, conjugation of pi-electrons, cyclic and obeying huckel’s rule. Huckel’s rule is containing (4n+2)π electrons, where n will be a non-zero integer.
Thus, benzene and all the derivatives of benzene are aromatic. Different names were given to different positions in aromatic compounds. The carbon that is directly bonded to the benzene ring can be called benzylic carbon.
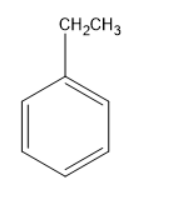
The above molecule is ethyl benzene, benzene consists of an ethyl group. Ethyl group consists of methylene and methyl group. As the methylene group is attached directly to the benzene ring, the carbon in the methylene group can be termed as benzylic carbon.
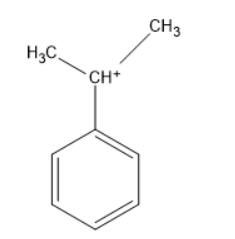
The above molecule is phenyl propyl carbocation, the carbocation carbon is directly attached to the benzene. The carbon bearing a positive charge can be called a carbocation. Thus, it is a benzylic carbocation.
Note:
In the compounds containing benzyl carbons the benzyl carbon is involved in the resonance stabilization with the aromatic ring. Thus, benzylic carbon is more reactive than the other positions in the aromatic compounds. In the second example, the carbocation will be involved in the resonance with the aromatic ring.
