Question
Question: What intermolecular forces are present in \( C{H_4} \) ?...
What intermolecular forces are present in CH4 ?
Solution
Hint : Intermolecular forces refer to those forces that mediate interaction between the molecules and they include forces of attraction and repulsion which are supposed to act between the atoms or other neighbouring particles like atoms or ions. Different types of intermolecular forces include ionic bonds, Vander Waals dipole-dipole interaction, hydrogen bonding and Vander Waals dispersion forces.
Complete Step By Step Answer:
The structure of CH4 is demonstrated below:
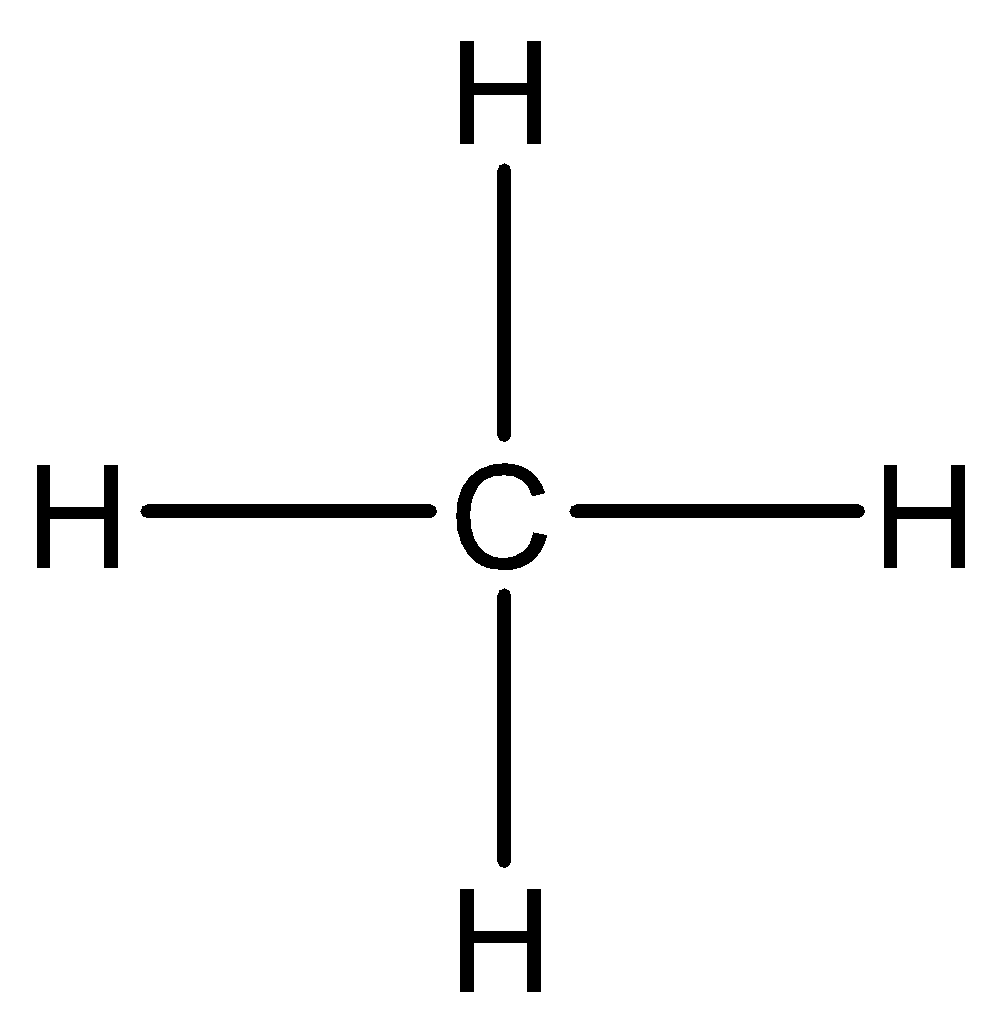
Let us look at different intermolecular forces one by one and find out which kind of forces are present in CH4 .
The weakest kind of intermolecular forces are Van Der Waals dispersion forces (temporary attractive forces) in which positive charge attracts electrons in an adjacent molecule. As we already know that when atoms or molecules are polarizable to a certain degree, some interactions (Vander Waals dispersion forces) occur from them when they are brought together as electrons are pushed about. All atoms and molecules possess these Van der Waals forces, so these forces are also present in CH4 .
Dipole-dipole interactions are present between the polar molecules. All of the outer atoms are the same with respect to the same dipoles and thus, dipole moments are in the similar direction i.e. towards the carbon atom making the overall molecule of methane CH4 as non-polar. As a result, methane comprises non-polar bonds, and is considered to be non-polar. Thus, CH4 does not possess dipole-dipole interaction.
Hydrogen bonding occurs in those molecules where hydrogen atom is bonded to any of the three most electronegative atoms (which include N , O or F ) such that hydrogen atom possess partial positive charge, and also a lone pair of electron must be present on the electronegative atom. Although CH4 contains four H atoms, hydrogen bonding is not present in CH4 as hydrogen is not bonded to N , O or F . The electronegativity of C ( 2.6 ) and H ( 2.2 ) are so close that C−H bonds are nonpolar and thus, no hydrogen bonding is observed in CH4 .
Hence, Vander Waals dispersion forces are found in CH4 .
Note :
Vander Waals dispersion forces are stronger in molecules which are not compact, but possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker.
