Question
Question: What happens when you pour some acetone on your palm?...
What happens when you pour some acetone on your palm?
Solution
The IUPAC name of acetone is propanone. It is extremely volatile and a flammable liquid and hence gets easily evaporated into its vapour form. It acts as an organic solvent and is miscible in water because it is polar due to the presence of carbonyl group (-CO-).
Complete step by step reaction:
-First of all we will see what acetone is.
Acetone is also known as propanone and is an organic compound with a molecular formula of (CH3)2CO or H3C−CO−CH3. It is the simplest ketone possible. Its structure is:
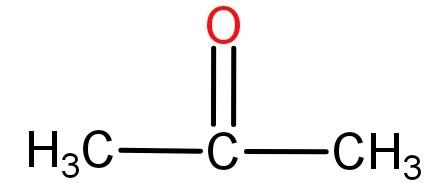
Acetone is colourless, extremely volatile, has a pungent odour and is a flammable liquid. It is polar and hence is miscible or soluble in water. It has the ability to act as an organic solvent.
-Acetone being extremely volatile and sublimate, gets converted into its vapour phase at a faster rate which increases the number of liquid molecules around it in the vapour phase. Thus when poured on our palm, acetone will absorb heat from our palms because our body temperature is high and then evaporates rapidly leaving a cooling sensation.
Hence when we pour acetone on our palm, it gives us a cooling sensation.
-Being a ketone acetone has the ability to undergo keto-enol tautomerism, aldol condensation and polymerisation at faster rates.
-Acetone has a large number of applications as follows. At an industrial level it is widely used as a solvent for many plastics and synthetic fibres. It is an active ingredient in nail polish remover and as paint thinner. It is also used in the medical field as a drug solvent and excipient, an anticonvulsant and for skin defatting.
-Acetone being highly flammable can be hazardous. When it burns it produces yellow bright flames. Hence the acetone used in industries has a small amount of water added to it which will inhibit its ignition and make it less hazardous.
-Hence we can conclude that by pouring acetone on our palms we feel a cooling sensation.
Note: Small amount of acetone is also present in the human body in the blood and in urine. It is both produced and disposed of in the human body via some metabolic processes. It is produced in diabetic patients in larger amounts. Introduction of ketogenic diets which can increase ketone bodies in our blood are extremely helpful to counter epileptic attacks in infants and children who suffer from refractory epilepsy.
