Question
Question: What happens when you pour some acetone on your palm?...
What happens when you pour some acetone on your palm?
Solution
Hint: The boiling point of acetone is 329 K. Think of which processes can easily be observed for solvents with such low boiling point.
Complete step-by-step solution:
Let us first learn about acetone in detail before studying this particular phenomenon.
Acetone (also known as propanone) is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colourless, volatile and flammable liquid with a recognizable and signature odour.
Acetone is miscible with water and serves as an important solvent in its own right, in industry, home, and laboratory. It is a common building block in organic chemistry. It is perhaps most commonly used as the active ingredient in nail polish remover and as paint thinner.
Let us now look at the structure of acetone/propanone.
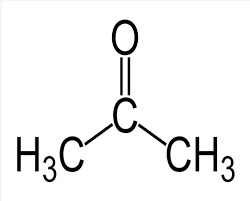
We observe a methyl group on either side of the functional carbonyl group, which is characteristic of ketones.
Now, let us look at this phenomenon in particular.
Acetone has a boiling point of 56oC which is a lot lesser than that of water. Additionally, due to the absence of forces of attraction (such as hydrogen bonding), acetone also tends to be incredibly volatile.
Thus, when poured on your palm, it absorbs the heat and evaporates quickly leaving a cool sensation thereafter. This procedure is not dangerous in the slightest and doesn't cause any harm to your skin.
Note: Understand the science behind the volatility of acetone and what that results in, instead of just trying to memorise this particular phenomenon. This phenomenon will help you understand many similar phenomena involving volatile solvents.
