Question
Question: What happens when \[{\text{C}}{{\text{H}}_3}{\text{Br}}\] is treated with \({\text{KCN}}\)?...
What happens when CH3Br is treated with KCN?
Solution
Alkyl halogens are derivatives of alkanes in which hydrogen is replaced with a halogen. SN2 reaction is undergone here. It is driven by the attraction between the negative charge of the nucleophile and positive charge on the leaving group.
Complete step by step answer:
Alkyl halides are organic molecules containing a halogen atom bonded to a sp3 hybridized carbon. Carbon-halogen bond of alkyl halides is polarized.
Alkyl halides undergo nucleophilic substitution reactions. Nucleophiles are the species with lone pairs (fully or slightly negative). They are strongly attracted to a region of positive charge. Weak nucleophiles do not have any negative charge. Strong nucleophiles contain negative charge and undergo SN2 reaction. Due to heterolysis of carbon-halogen bonds, nucleophiles form new bonds with carbon atoms by replacing halogen. Leaving group is the substituent that leaves substrate. It leaves in stable condition. E.g. Br,Cl,I.
SN2 reaction is called bimolecular nucleophilic substitution reaction. It is a single step in primary carbon. No intermediates are formed, but transition states are formed. And the configuration is inverted.
Here, bromo methane reacts with potassium cyanide to give cyano methane and potassium bromide.
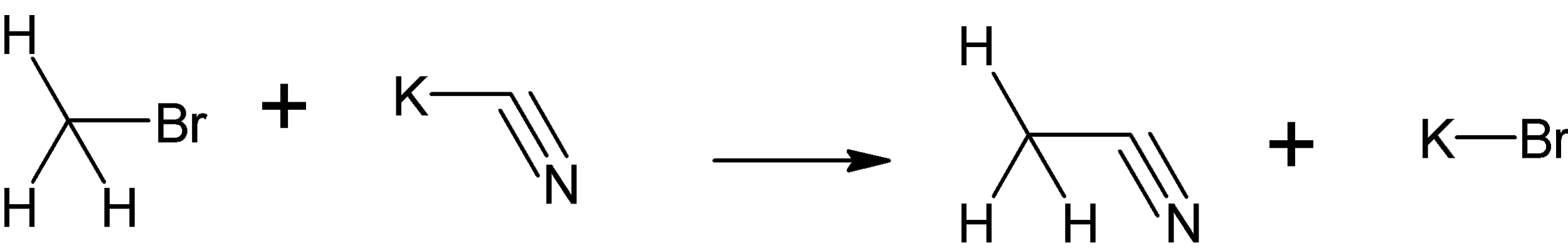
When the nucleophile attacks the carbon with bromine, it forms a transition state which is a combined product which contains both bromine and cyano groups.
Hence when CH3Br is treated with KCN, CH3CN and KBr is formed.
Note:
The factors that affect the SN2 reaction are steric effect, nucleophile, solvent effect and leaving group. The reactivity order in this reaction is CH3>1∘>2∘>3∘. Nucleophilicity increases from left to right across the period and it increases down the group.
