Question
Question: What happens when (i) Chlorobenzene is treated with \[C{l_2}/FeC{l_3}\] (ii) Ethyl chloride is ...
What happens when
(i) Chlorobenzene is treated with Cl2/FeCl3
(ii) Ethyl chloride is treated with AgNO2
(iii) 2-bromopentane is treated with alcoholic KOH?
Write the chemical equation in support of your answer.
Solution
In the first reaction, when chlorobenzene reacts with Cl2/FeCl3, it will undergo nucleophilic substitution reaction and give multiple products.
In the second reaction, Ethyl chloride is reacted with AgNO2. This reaction is the Victor Mayer method.
In the third reaction, 2-bromopentane is treated with alcoholic KOH, will follow Saytzeff’s rule and undergo β− elimination.
Complete step by step answer:
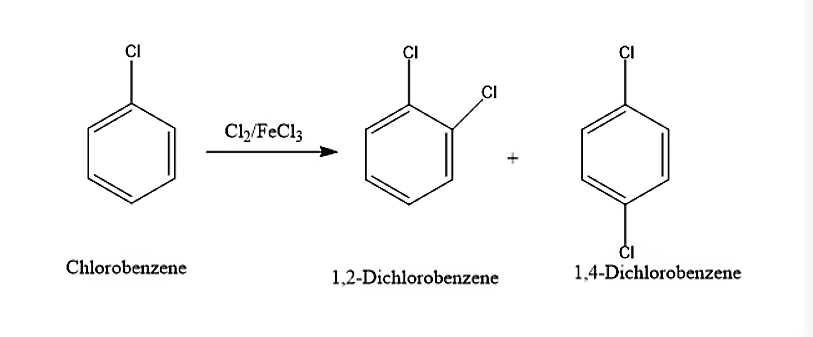
(i) When Chlorobenzene is treated with Cl2/FeCl3, it will lead to the formation of both ortho and para isomers. The ortho isomer will be 1,2-Dichlorobenzene and the para isomer will be 1,4-Dichlorobenzene. The reaction is given below
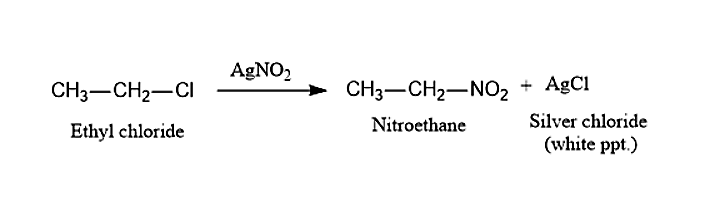
(ii) When Ethyl chloride, CH3CH2Cl is treated with Silver Nitrite, AgNO2it will lead to the formation of Nitroethane and white precipitate of Silver chloride, AgCl. This process is known as Victor Meyer Method.
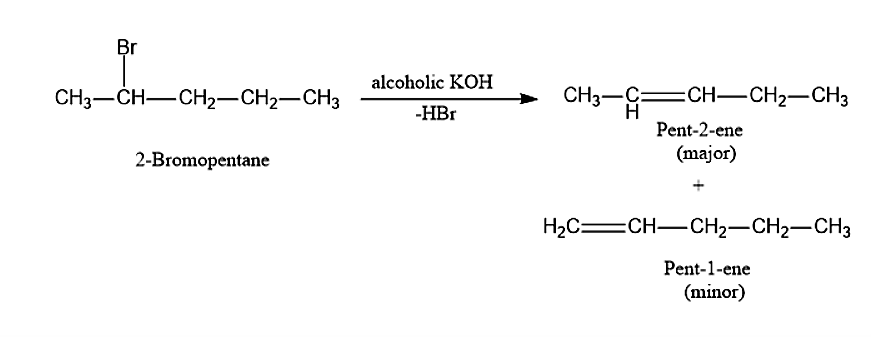
(iii) When 2-bromopentane is treated with alcoholic KOH it will follow Saytzeff’s rule and will undergo β− elimination. This reaction will give two products. Among that one will be major and the other will be minor. The major product in this reaction is Pent-2-ene and the minor product will be Pent-1-ene.
Therefore,
(i) When Chlorobenzene is treated with Cl2/FeCl3, it will give 1,2-Dichlorobenzene and 1,4-Dichlorobenzene.
(ii) When Ethyl chloride is treated with AgNO2, it will give Nitroethane.
(iii) When 2-bromopentane is treated with alcoholic KOH, it will give Pent-2-ene as the major product and Pent-1-ene as the minor product.
Note: According to the Saytzeff’s rule, when dehydrohalogenation reaction takes place and the most stable product will be that alkene i.e., 2-pentene, which has largely substituted alkene group in it. The less substituted alkene will be the minor product and also called the Hofmann product.
