Question
Question: What happens when ethyl chloride is treated with \[AgN{O_2}\] ? Write structure of the product?...
What happens when ethyl chloride is treated with AgNO2 ? Write structure of the product?
Solution
Ethyl chloride is an ethane molecule having a chloride group attached to one of its two carbon atoms in place of a hydrogen atom. On the other hand AgNO2 is known as silver nitrate and is an important chemical compound in many reactions.
Complete answer:
AgNO2 is an important and commonly used chemical compound.
So, we will see its reaction with the ethyl chloride, so let’s write the balanced chemical equation of the reaction between the ethyl chloride and the silver nitrate, AgNO2 :
CH3−CH2Cl+AgNO2→CH3−CH2NO2+AgCl
So, as we can see from the above reaction that when ethyl chloride reacts with the silver nitrate , it results in the production of ‘Nitro ethane ‘ and also in addition to it a silver colored compound – silver chloride is also produced as a by-product.
So, it is clear from the above discussion that ‘nitro ethane’ is produced in reaction of ethyl chloride with silver nitrate.
So, now also let’s have a look at the structural formula of the ‘nitro ethane’ molecule.
Therefore, the structure is:
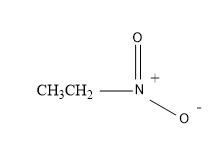
So from the above figure, the structure of the nitro ethane is crystal clear and is easy to understand.
Therefore, the correct answer is ‘silica’.
Note:
Also from the above discussion and from the structure of the ‘Nitro ethane’ we can say that the reaction between ethyl chloride and silver nitrate follows the SN2 mechanism of attack. Also note that the silver chloride is also produced as the silver precipitate in the above reaction.
