Question
Question: What happens when anisole is treated with \(C{{H}_{3}}Cl/anhydrous\ AlC{{l}_{3}}\)? Write a chemical...
What happens when anisole is treated with CH3Cl/anhydrous AlCl3? Write a chemical equation in support of your answer.
Solution
Anisole is an organic compound in which the benzene is connected with the methyl group with the help of the ether group. Anisole reacting with CH3Cl/anhydrous AlCl3 is called Friedel Craft alkylation reaction.
Complete step by step answer:
Anisole is an organic compound in which the benzene is connected with the methyl group with the help of the ether group. Anisole compound comes under the aromatic ether group. The formula of anisole is C6H5−OCH3. The structure of Anisole is given below:
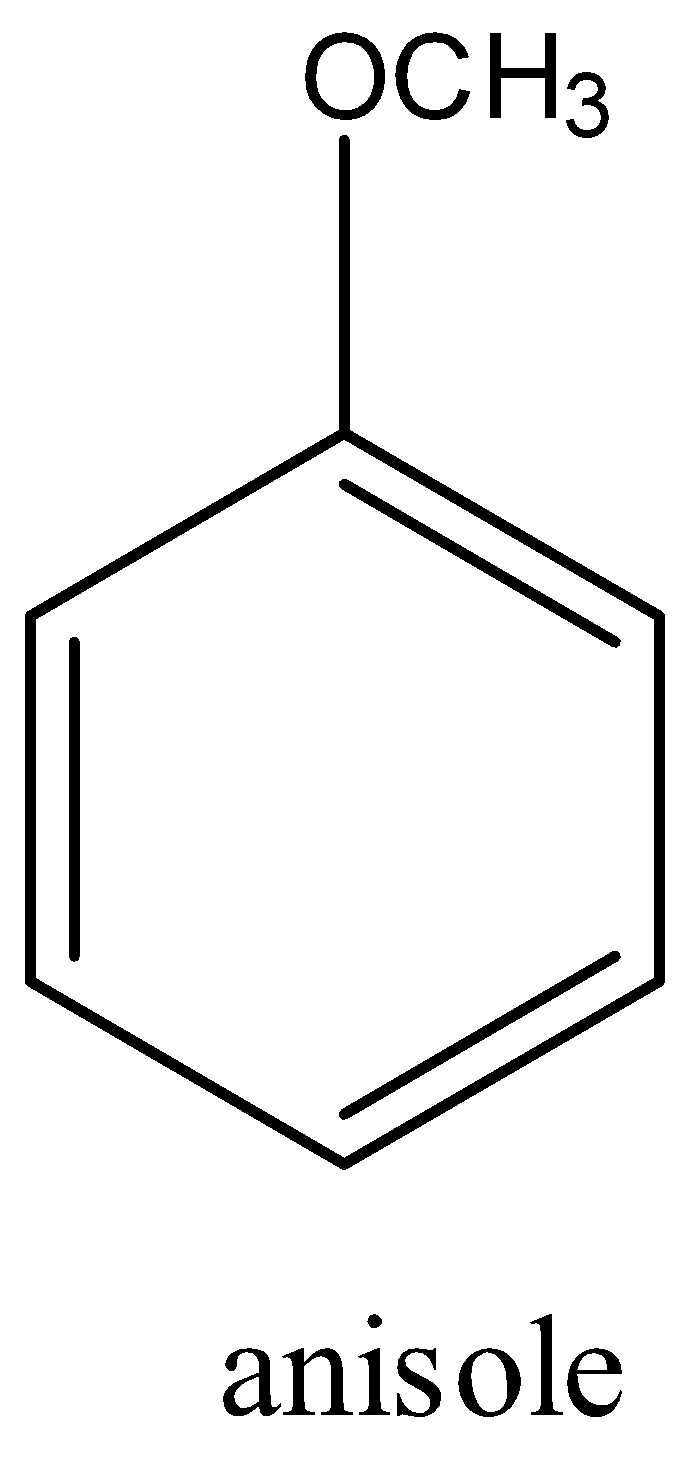
The IUPAC name of anisole is methoxybenzene.
The chemical reaction of anisole follows the Electrophilic substitution reaction procedure.
CH3Cl/anhydrous AlCl3 is called methyl chloride in the presence of anhydrous aluminium chloride. It is converted into methyl cation and chloride anion. In this methyl cation is the electrophile.
So, when anisole is reacted with CH3Cl/anhydrous AlCl3, there is a substitution of a methyl group at ortho and para position of the benzene. The product formed when the methyl group attaches to the ortho position is called o-methyl anisole and the product formed when the methyl group attaches to the para position is called p-methyl anisole. The reaction is given below:
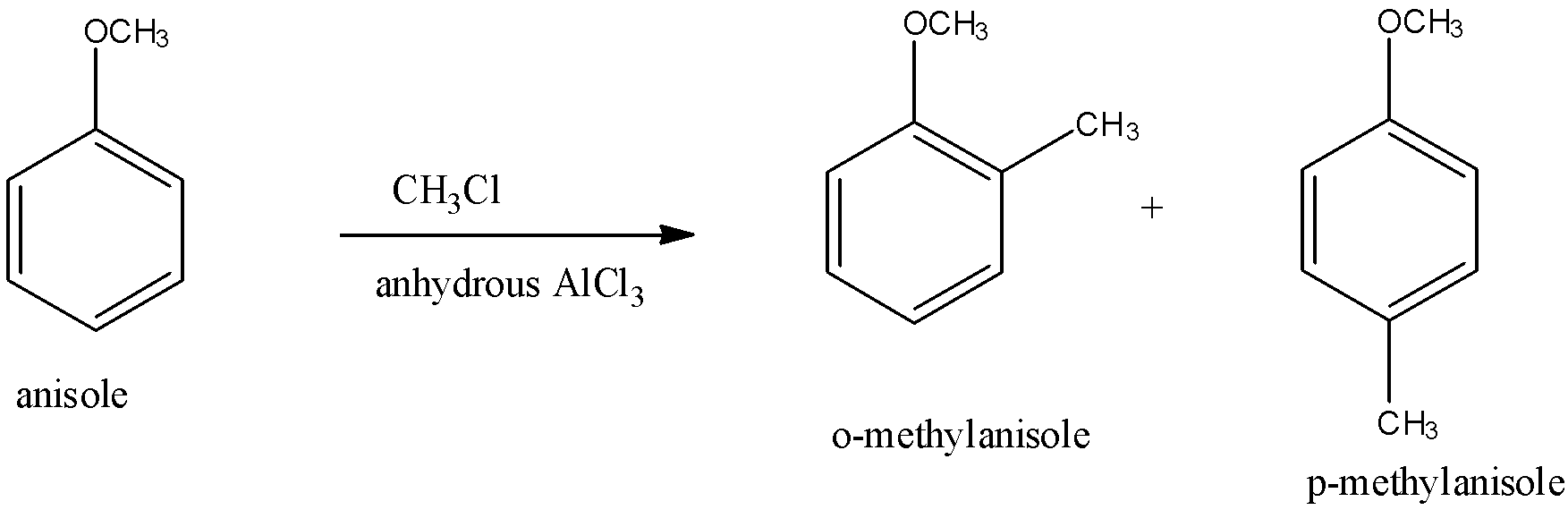
From both the products, p-methyl anisole is the major product because of the symmetry of the molecule. This reaction is called the Friedel-Craft alkylation reaction.
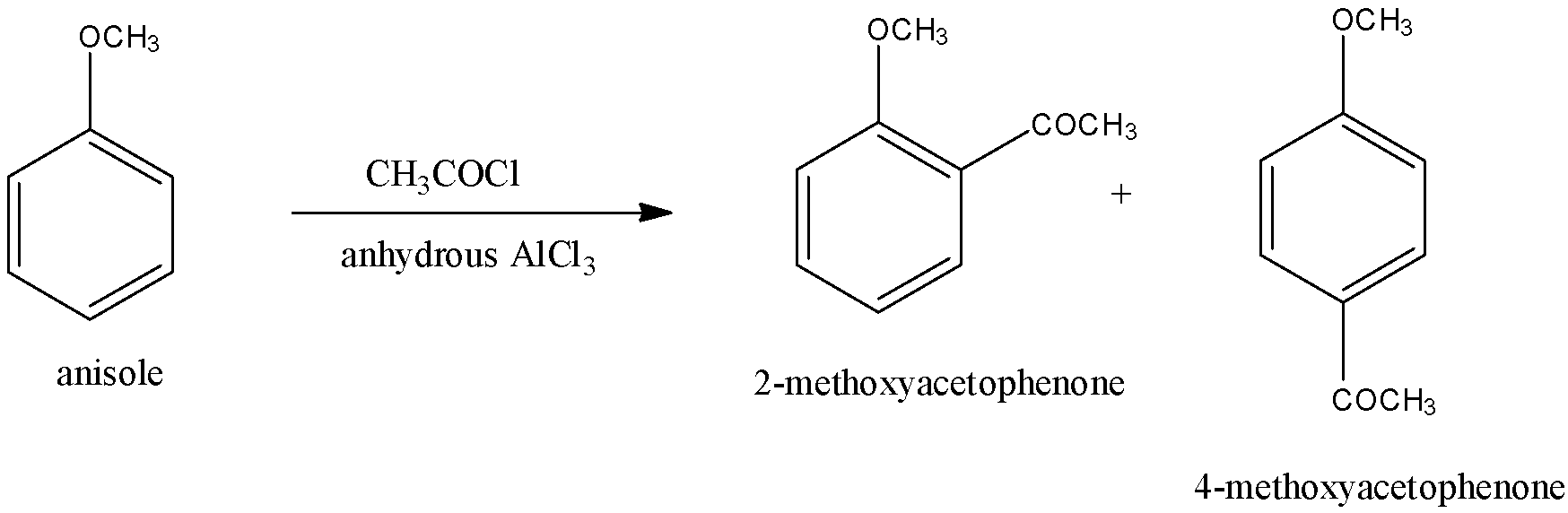
Note: When the reactant is taken CH3COCl/anhydrous AlCl3, 2-Methoxyacetophenone, and 4-Methoxyacetophenone is formed. It also follows the electrophilic substitution reaction. In CH3COCl/anhydrous AlCl3, COCH3 acts as the electrophile. This reaction is called Friedel-Craft acylation. 4-Methoxyacetophenone is the major product. The reaction is given below: