Question
Question: What happens when aniline reacts with the following? Bromine water...
What happens when aniline reacts with the following?
Bromine water
Solution
As we know that in chemistry, equations play a major role. In the reaction three things are main. There are reactants, products and reaction conditions. The reactants are always on the left side of the equation. If more than one reactant in the equation means by using plus sign. The products are always on the right side of the reaction. Something here also follows. More than one product by using plus sign. In between reactant products are used arrows to find the reaction direction in the equation.
Complete answer:
In organic chemistry benzene is one of the important compounds. It is one of the aromatic compounds. The molecular formula of benzene is C6H6. Aniline is one of the derivatives of benzene. The molecular formula of aniline is C6NH7.
We can draw the structural formula of aniline as,
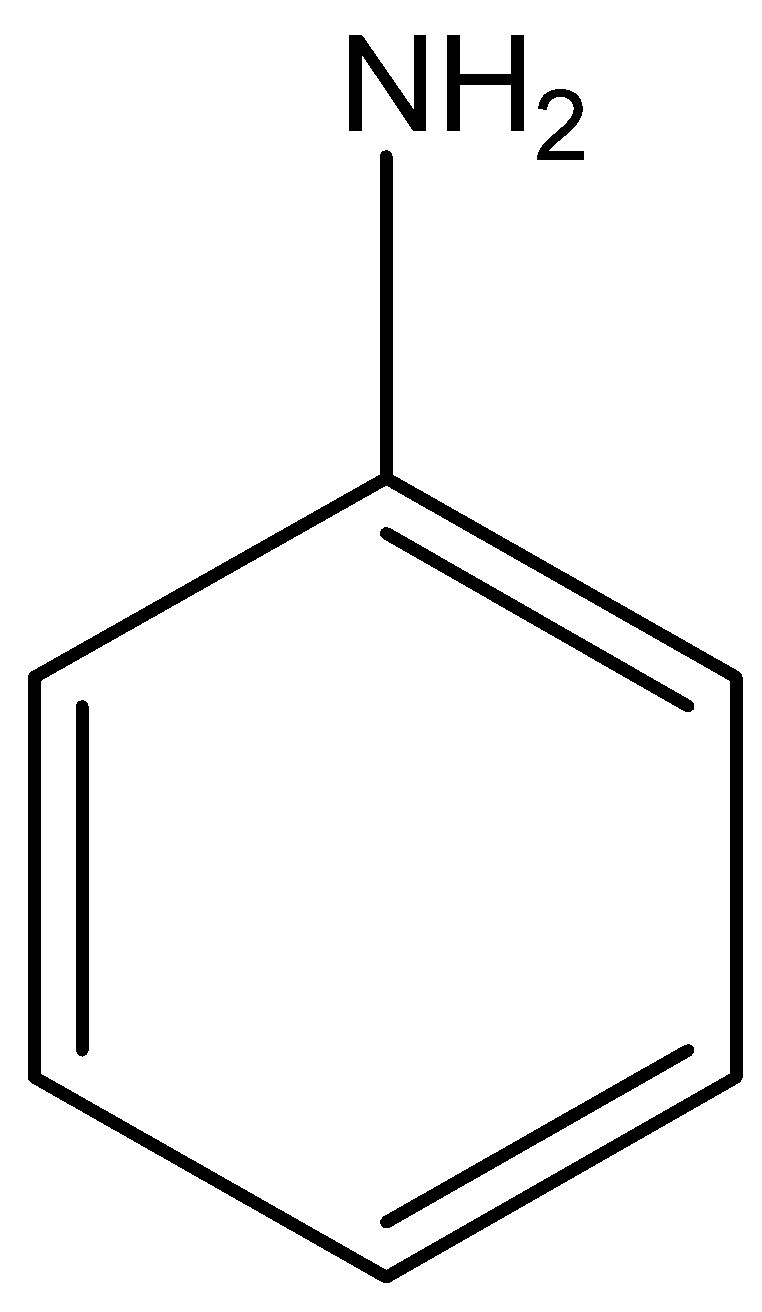
Aniline when treated with bromine in water gives a product of 2,4,6- tribromo aniline. It is white precipitate.
The reaction for the above discussion is given below,
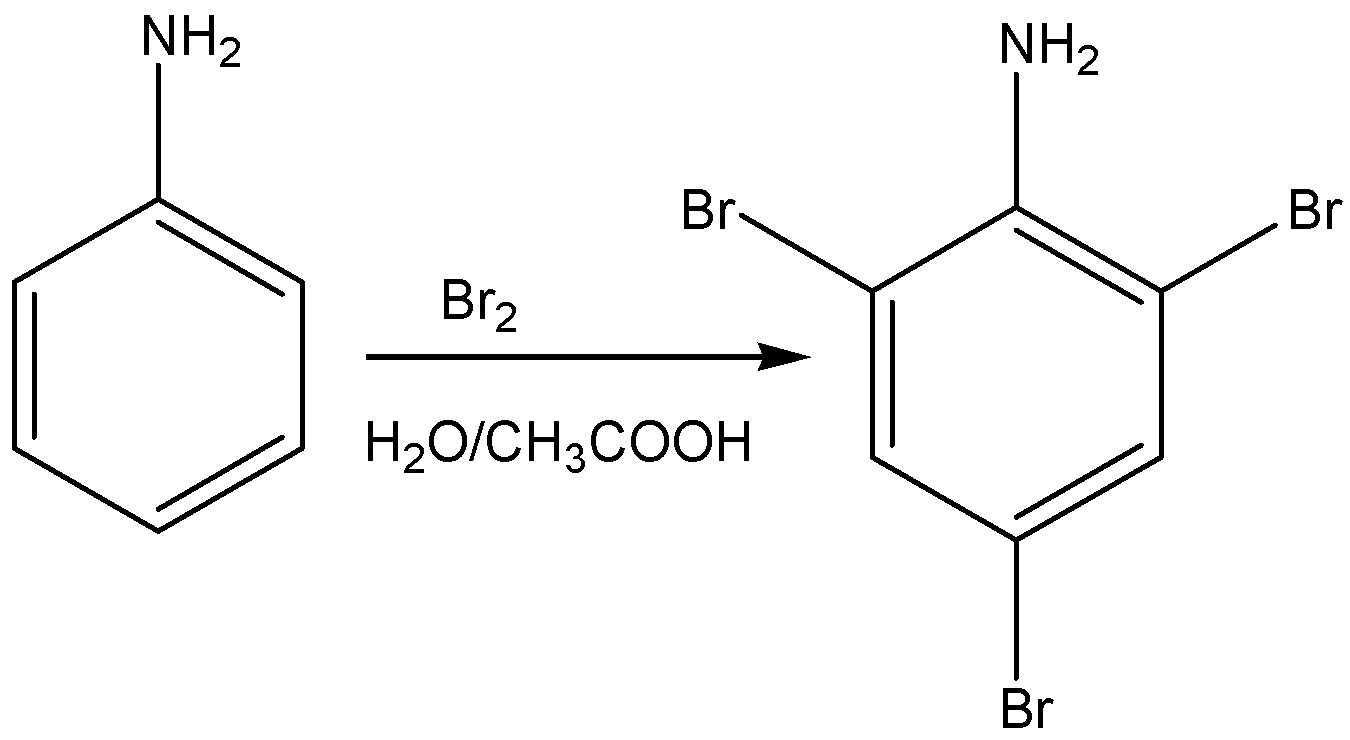
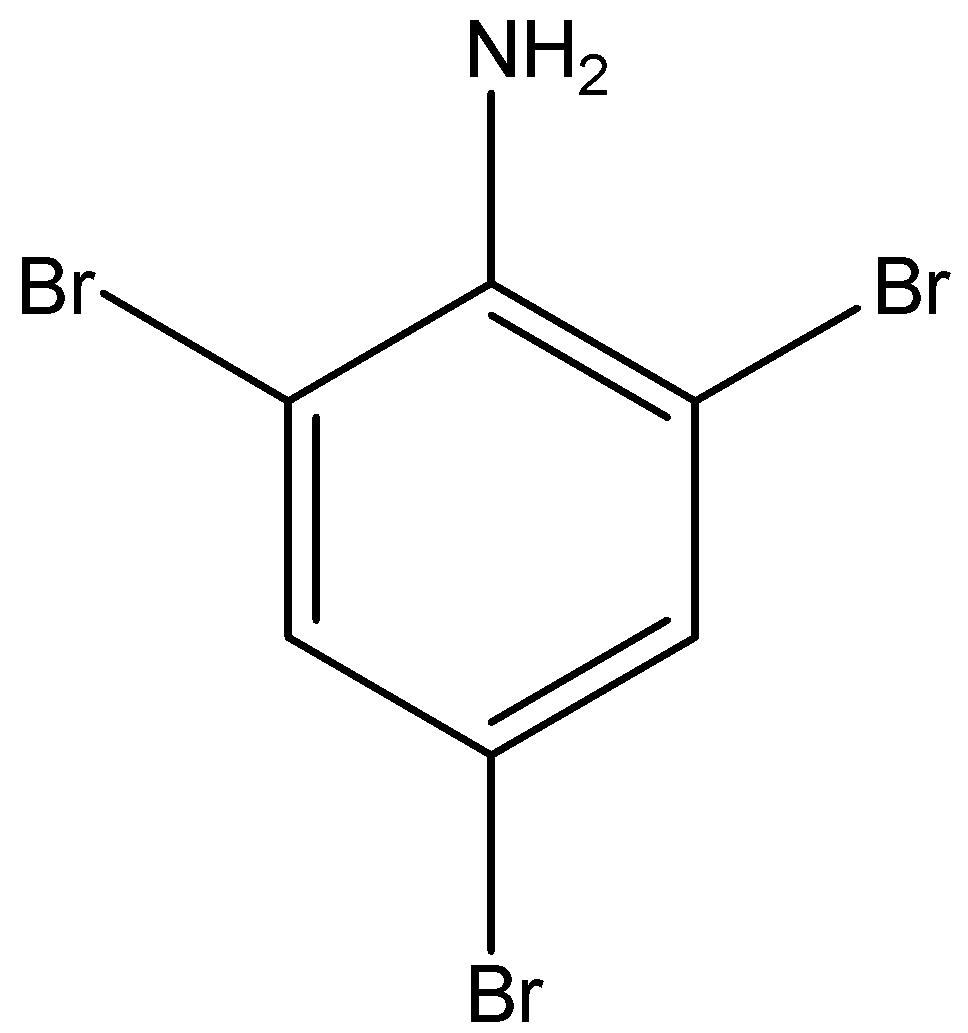
The structural formula of 2,4,6- tribromo aniline is
If we want a certain position, bromination means we first do acylated aniline to get acetanilide. Acetanilide is treated with bromine in acetic acid mainly gives p-Bromoacetanilide. This p-Bromoacetanilide undergoes hydrolysis to give p-Bromoaniline.
The reaction for the above discussion is given below,
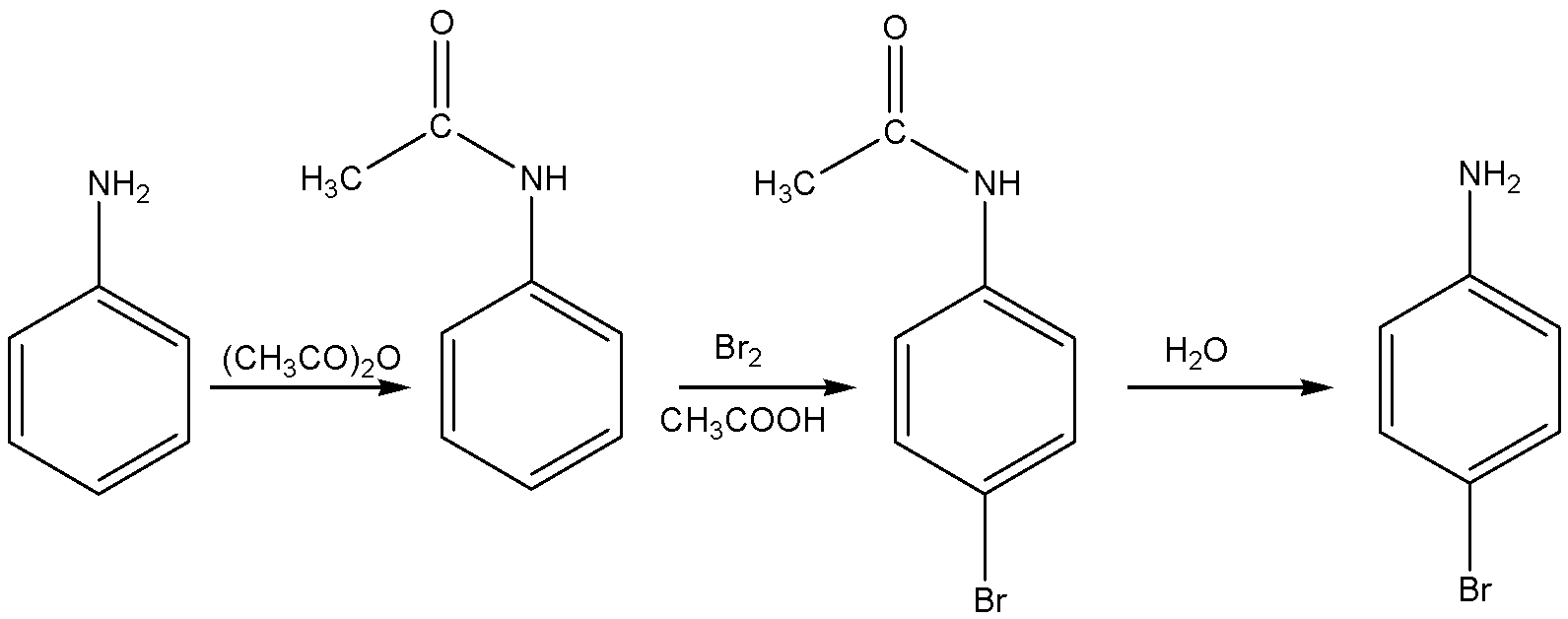
The structural formula of p-Bromo aniline is
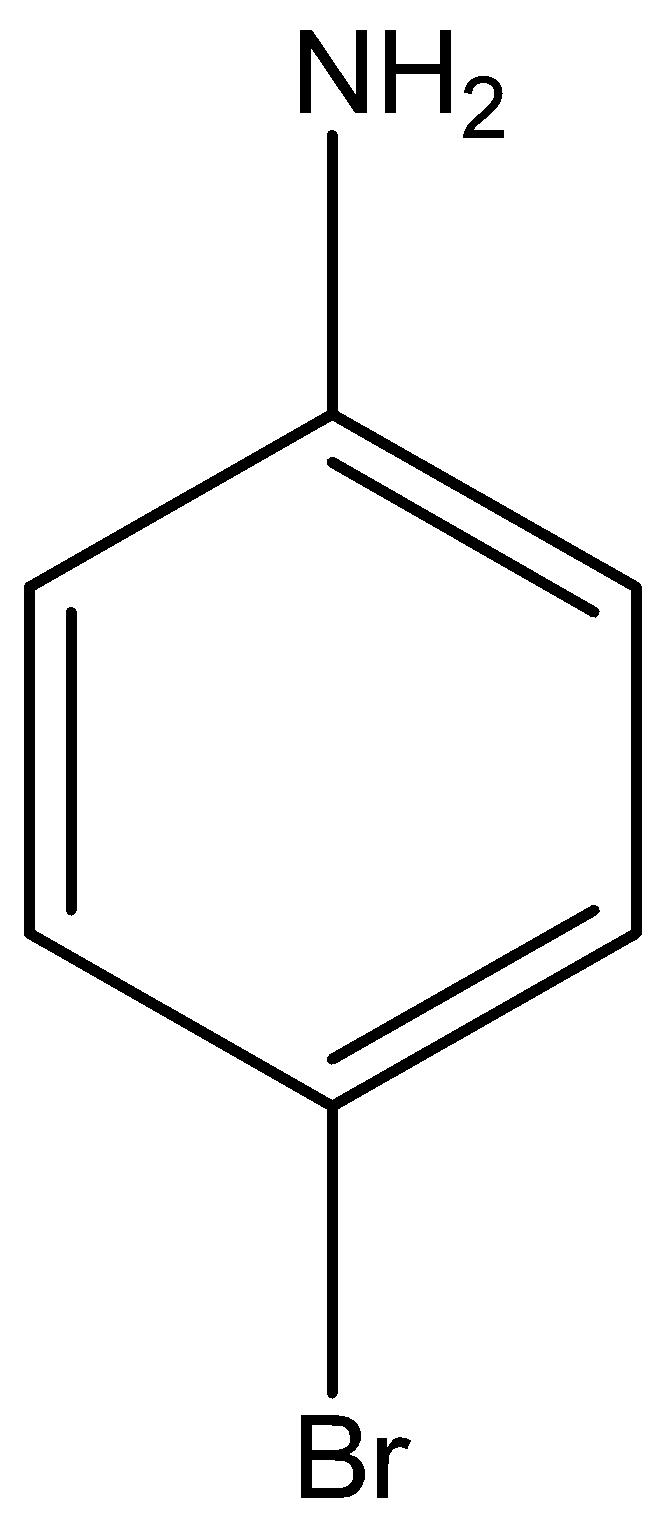
Acetanilide is treated with bromine in acetic acid mainly gives p-Bromoacetanilide.
According to the above discussion we conclude aniline when treated with bromine in water gives a product of 2,4,6- tribromo aniline.
Note:
In balanced chemical reaction wise, we predict the side product of the reaction. In some cases we are not able to attain the balance by changing the mole. In that case we use the number of countable ions in the product and reactant side of the chemical reaction. In chemistry, redox reaction is one of the types of major reactions. In this redox reaction two methods are used to attain the balanced chemical reaction. There are ion-electron methods and the oxidation number method.
