Question
Question: What happens when acetic acid is treated with \[Zn\] metal?...
What happens when acetic acid is treated with Zn metal?
Solution
Zinc is a reactive metal which leads to displacement / substitution reaction when reacted with acetic acid. Hydrogen is less reactive than Zinc. Zinc is slightly brittle at room temperature and has a blue-silvery appearance.
Complete step by step answer:
First of all let us understand something about acetic acid. Acetic acid has an IUPAC name ethanoic acid with chemical formula C2H4O2 . It’s structure is as below:-
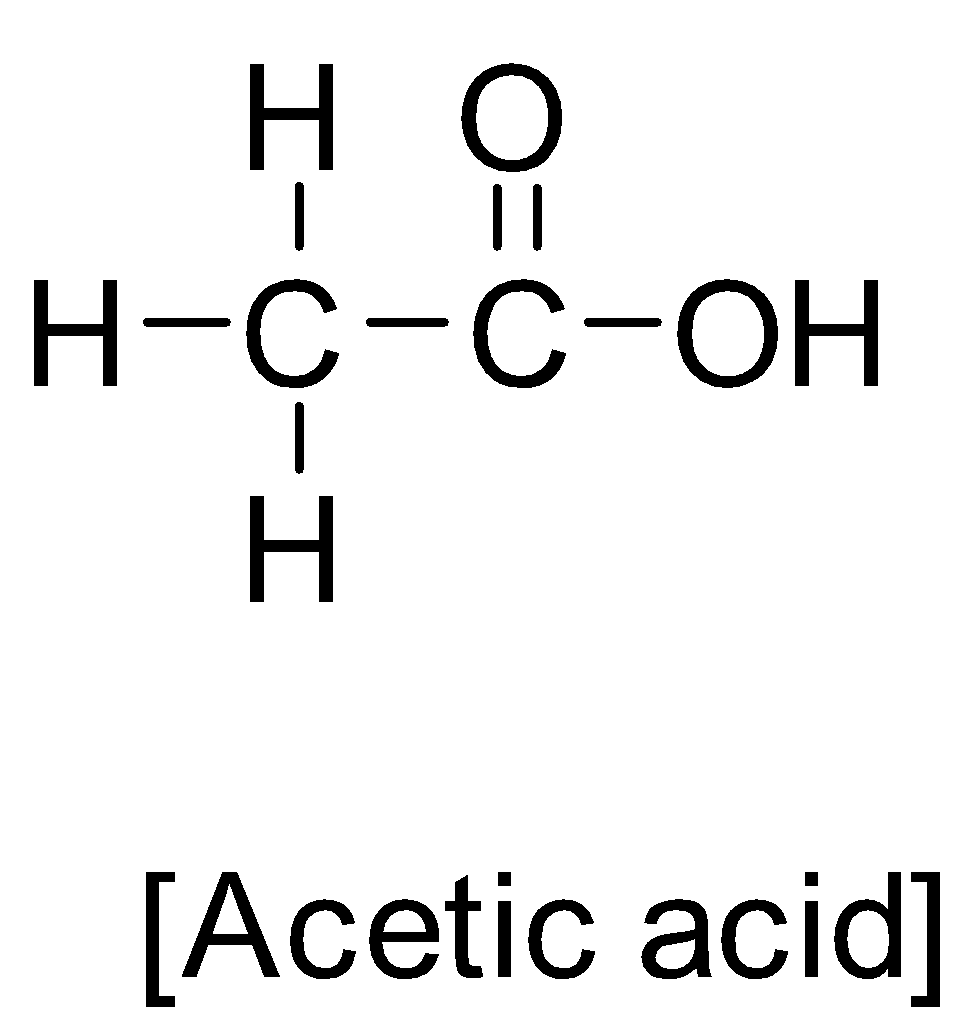
Acetic acid is a colourless liquid organic compound. It is an important industrial chemical and chemical reagent. Acetic acid has a pungent small and has sour taste. It is considered as a weak acid because it did not dissociate completely in water. It is used in making vinegar. Acetic acid is used in the production of esters. Moreover it is also the main component in the formation of acetic anhydride. Acetic acid is also used as an organic solvent/polar solvent. Acetic acid injection is used to treat cancer. Acetic acid is also an antiseptic acid that has a corrosion nature which can cause spinal damage.
Zinc is a reacting element with an atomic number of 30. Zinc has five stable isotopes and is the 24th most abundant element in earth’s crust.
When zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of H2 gas. The reaction tapes place as
Zn+2CH3COOH→Zn(CH3COO)2+H2
Note: This reaction does not need any catalyst as the zinc is the transition element and can act as self-catalyst. Moreover in the above reaction, acetic acid having corrosive nature gently corrodes the zinc surface.
