Question
Question: What happens when? (1) \[PC{l_5}\] is heated (2) \({H_3}P{O_3}\) is heated? Write the reactio...
What happens when?
(1) PCl5 is heated
(2) H3PO3 is heated?
Write the reaction involved.
Solution
Try and draw the structure of PCl5 and focus on the bonds and also the fact that on heating PCl5 undergoes an equilibrium reaction .
For H3PO3 focus on the various oxidation states possible for P atoms.
Complete step by step answer:
(1)PCl5 is heated:
The phosphorus atom in PCl5 has a hybridisation of sp3d and thus it has a triangular bipyramidal structure.
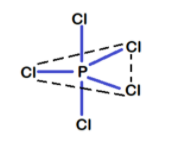
In this type of structure 3 bonds are equatorial (in a plane) and 2 are axial bonds (one above the plane and other below). The 2 axial bonds are at an angle of 90∘ to the 3 equatorial bonds. While the 3 equatorial bonds are at an angle of 120∘ to each other. In this way the axial bonds are nearer to the equatorial bonds. Hence axial bonds experience greater repulsion from both sides leading to the elongation of these axial bonds.
So, axial bonds (219pm) are longer than the equatorial bonds (204pm). Due to the repulsion experienced by the axial bonds they have lesser stability than the equatorial bonds.
So when PCl5 is heated the 2 unstable axial bonds break, causing PCl5 to decompose into PCl3 and Cl2 .
This reaction can be written as:
PCl5ΔPCl3+Cl2
(2)H3PO3 is heated:
When H3PO3 is heated it undergoes a disproportionation reaction and forms H3PO4 andPH3.
The oxidation state of P in H3PO3 is +3, in H3PO4it is +5 and in PH3 it is -3. Here we can see that the oxidation number of P is both decreasing and increasing which means that it undergoes both reduction and oxidation.
So, H3PO3 undergoes a disproportionation reaction.
This reaction can be written as:
4H3PO3Δ3H3PO4+PH3
Note: While doing such questions always be careful while describing the structures and bonds. Also the oxidation states should be calculated with precision and also do not get confused between phosphoric acid (H3PO4) and orthophosphorous acid (H3PO3).
