Question
Question: What happened when: \(B{{F}_{3}}\) is reacted with ammonia?...
What happened when:
BF3 is reacted with ammonia?
Solution
The BF3 is a Lewis acid i.e. an electron deficient species and NH3 is a Lewis base, a species which consists of lone pairs that can be donated for the formation of bonds.
- NH3 molecule consists of a lone pair of electrons .
Complete Solution :
So here in the question we are asked to predict what happens when NH3 and BF3 reacts together.
Before predicting the product let’s see some characteristics of ammonia and boron trifluoride.
So in BF3 molecule B is the central atom and we know that theBF3 is a Lewis acid i.e. an electron deficient species.
- The atomic number of B is 5 and has an electronic configuration:
E.CofB=1s22s22p1
- The valency possessed by the B atom is +3.
- The hybridization of BF3 molecule is sp2 and hence having a planar structure.
- In NH3 molecule, N is the central atom having the atomic number 7 and the electronic configuration is,
E.CofN=1s22s22p3
Ammonia is a sp3 hybridized molecule in which one lone pair is present and hence having a trigonal pyramidal structure.
So as we discussed earlier BF3 is a Lewis acid and requires two electrons for B to obtain the octet configuration and NH3 consists of one lone pair of electron and it acts as the Lewis base according to Lewis theory.
Hence the NH3 molecule donates its lone pair of electrons to the BF3 molecule and forms a coordinate bond.
So the ammonia and boron trifluoride on reactions forms an adduct.
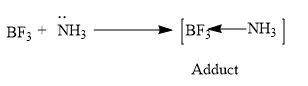
NH3 The hybridization of this adduct formed is sp3 hybridized adduct.
Note: The hybridization of a molecule is calculate using the formulae,
steric number = No. of bps + No.of lps
Bps refers to bond pairs and lps refers to lone pairs and the steric number obtained will give an idea about the hybridization of the molecule.
