Question
Question: What does the distillation of phenol with Zinc Dust result in? (A) \({{C}_{6}}{{H}_{6}}\) (B) \...
What does the distillation of phenol with Zinc Dust result in?
(A) C6H6
(B) C6H5−C6H5
(C) C6H12
(D) C6H5−O−C6H5
Solution
Hint : A common name for this reaction is the Baeyer reduction of aromatic oxygen-containing compounds. Here, reduction of phenol will occur.
Complete step by step solution :
Let us break this reaction down step-by-step to help facilitate better understanding of the concept.
- Let us now go through the mechanism of this reaction to understand how the formation of Benzene and Zinc Oxide (ZnO) really takes place.
- Zn shows an oxidation state of +2.
- The phenol turns into phenoxide ion and the proton that is released accepts an electron from Zn forming H radical.
- Also because of the heating there is homolytic fission of C of phenyl ring and O-
- Thus, the O-atom formed accepts an electron from Zn and forms oxide ion.
- In this way zinc metal forms zinc oxide and phenyl radical produced here forms a bond with hydrogen radical.
- However, the yield of this reaction is lower.
- The full mechanism is shown step-by-step in the following figure:
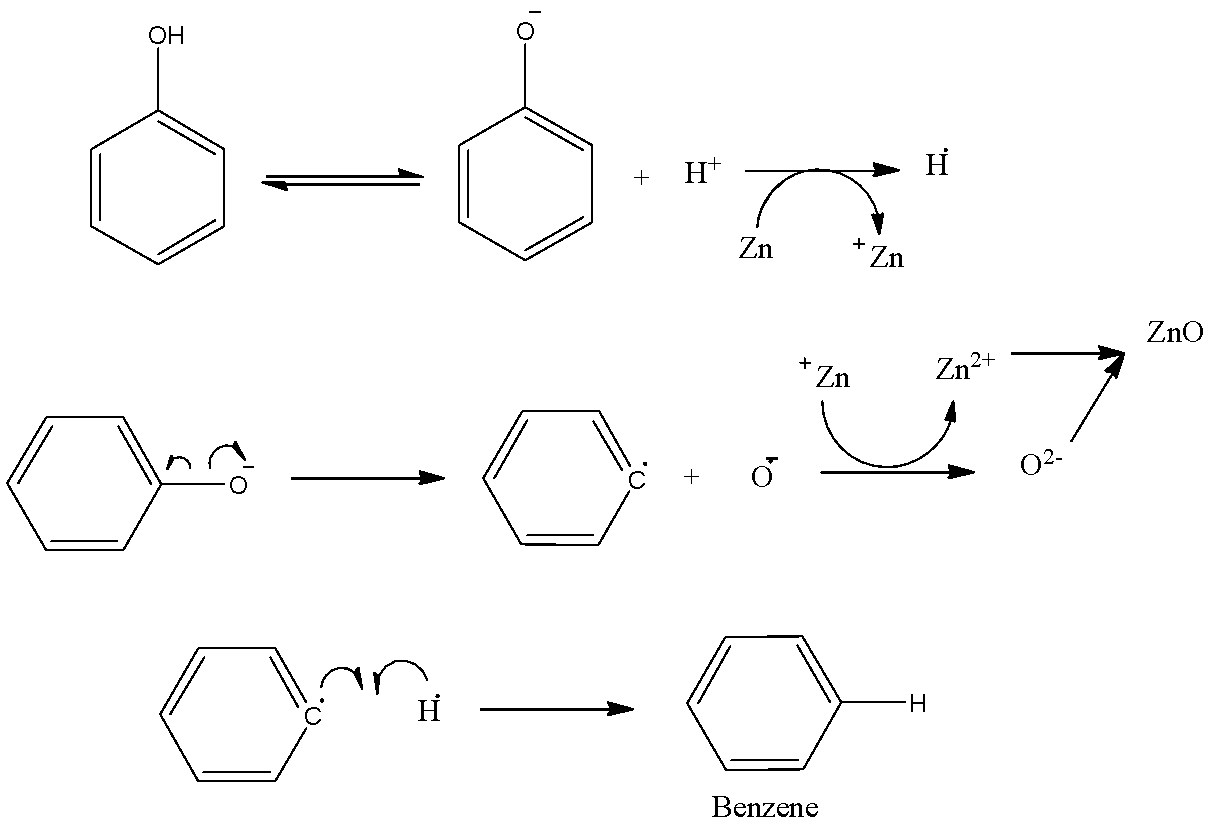
- Observe that the formation of C12H10 and H2 is also possible due to the fusion of the benzene and the hydrogen radicals with another one of the same kinds.
- The equation of the given reaction is as follows:
C6H5−OHZnOC6H6
So, the correct answer is “Option A”.
Note : Do not assume that Zinc oxide will oxidise phenol as it has an oxygen atom in it. Remember that actually it is used as a reducing agent. Remember that Zinc oxide cannot reduce carbon-carbon double bonds. It can only reduce carbon-oxygen single bonds.
