Question
Question: What does phenol form by reaction with \(HCN\) and \(HCl\)....
What does phenol form by reaction with HCN and HCl.
Solution
Phenol is an aromatic compound in which electrons are delocalized. When phenol is reacted with the mixture of HCNand HCl it forms aldimine as the intermediate. The catalyst we use for this reaction is ZnCl2. Thus we do hydrolysis of the intermediate product to get the final product.
Complete answer:
Phenol is an aromatic compound which contains hydroxyl groups. The mixture of HCNand HClis reacted with phenol in the presence of a catalyst known as zinc chloride. Further an intermediate product is formed which on hydrolysis yields us p−hydroxybenzaldehyde. The whole reaction will take place in the following steps:
1.In the first stage of reaction, the mixture of hydrochloric acid and hydrogen cyanide is reacted to form a complex in the presence of the zinc chloride.
HCl + HCN ZnCl2 Cl - CH = NH
The formed complex will further react with the phenol and form an intermediate product.
2. Now the given complex will react with phenol and produce an intermediate product known as aldimine. Then the aldimine so formed will undergo hydrolysis.
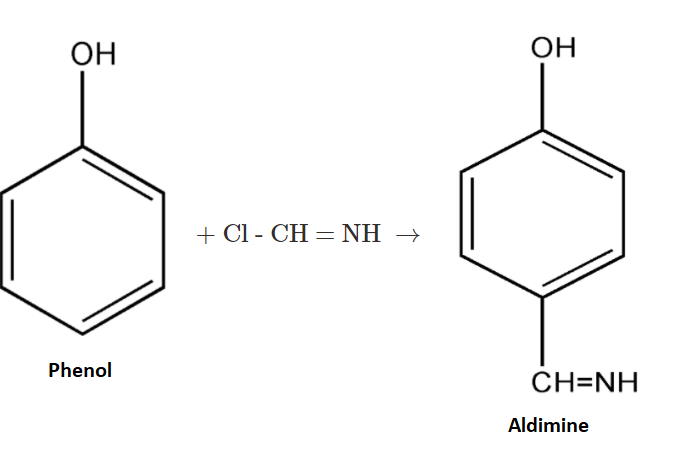
3.Now the formed intermediate, Aldimine, is further undergoing hydrolysis with H3O+. On hydrolysis of the above intermediate we get p−hydroxybenzaldehyde as our main product.
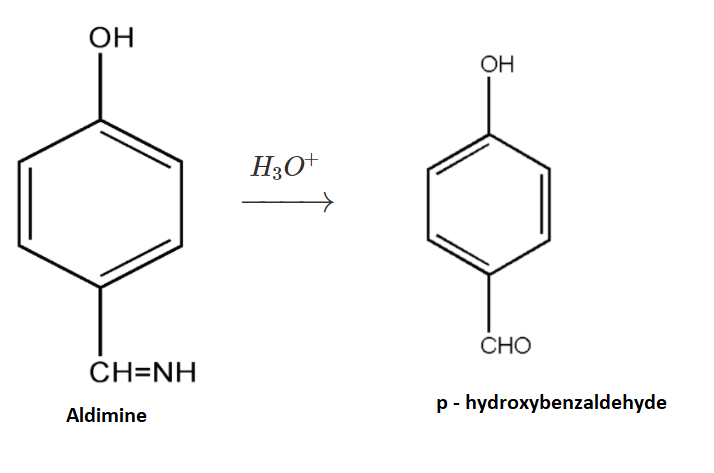
The product so formed is p−hydroxybenzaldehyde. The reaction is also known as the Gattermann Reaction used for synthesis of aromatic aldehydes.
Note:
The intermediate formed during the reaction is a result of zinc chloride. Here zinc chloride acts as a catalyst. We can use others like Aluminium Chloride in place of Zinc Chloride. On hydrolysis there is addition of oxygen and an amine group if formed as a by-product.
