Question
Question: What does pH stand for? What does a pH scale indicate?...
What does pH stand for? What does a pH scale indicate?
Solution
pH is the measure of the acidity or the basicity of any aqueous solution. With the help of pH scale we can predict the nature of a substance whether it is acidic in nature or basic in nature. The pH scale has a neutral point below which a substance is considered to be acidic and above which it is considered to be basic.
Complete answer:
Basically the ‘pH’ phrase is made of two alphabets which are ‘p’ and ‘H’ respectively. Therefore the alphabet ‘p’ represents the potential or the power and the alphabet ‘H’ represents hydrogen ions. Thus it is the measure of the potential of the hydrogen ions in a solution. Mathematically it can be represented as the negative logarithmic value of hydrogen ion concentration which can be shown as:
⇒pH = −log[H+]
pH scale is the measurement scale which used to tell us about the nature of aqueous solution. The pH scale ranges from 0−14 and here 7 is called a neutral point. The substances having pH less than seven are considered to be acidic in nature and substances having pH more than seven are considered to be basic in nature. The pH scale can be represented as:
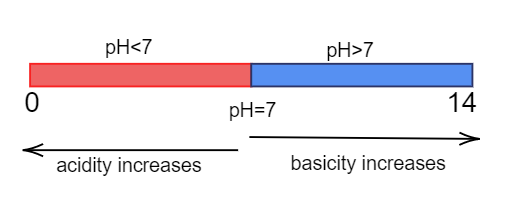
Note:
It must be noted that the neutral point lies at a pH equal to seven. As we move towards the right of the neutral point then basicity increases and acidity decreases. Similarly when moving left of the neutral point then acidity increases and basicity decreases. Like pH we can also define pOH which is a measure of the potential of hydroxide ions.
